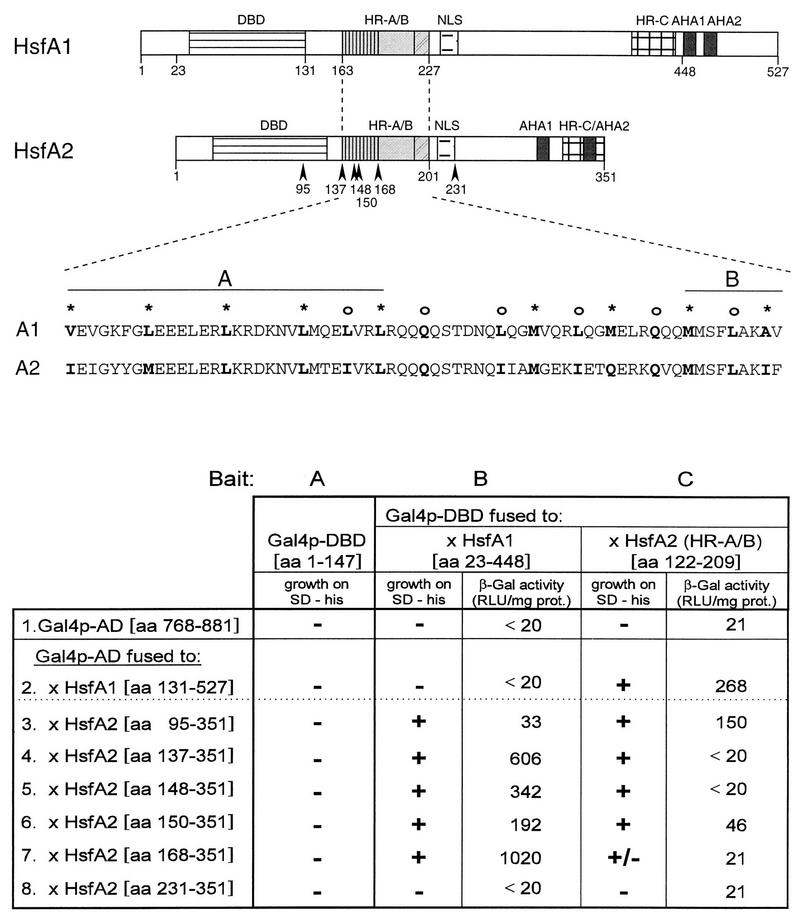
FIG. 9.
Yeast two-hybrid test for interaction between HsfA1 and HsfA2. (Top) Basic structure of the two tomato Hsfs. AHA1 and AHA2, activator modules (for details, see reviews by Nover et al. [30] and Nover and Scharf [27]). Sequence details for the HR-A/B regions with the conserved heptad repeat pattern of hydrophobic amino acid residues are given. For the two-hybrid constructs, we used the usual N-terminal (aa 1 to 147) and C-terminal (aa 768 to 881) parts of the yeast Gal4 activator protein (see Materials and Methods). (Bottom) For the two-hybrid test, the baits were composed of the DBD or HR region of Gal4p (aa 1 to 147) alone (bait A) or fused to the indicated parts of the tomato Hsfs, i.e., HsfA1 (aa 23 to 448 [bait B]) and HsfA2 (aa 122 to 209 [bait C]). The activators combined with them were Hsf fragments fused to the C-terminal Gal4p activator domain (Gal4p-AD) (samples 2 to 8). Interaction is indicated by growth (+) or nongrowth (−) of the cultures on His-free synthetic dextrose minimal (SD) medium and by the activity measured in the lacZ assay (RLU per second and milligram of protein [see Materials and Methods]). β-Gal, β-galactosidase; prot., protein.