Summary
This Food and Drug Administration (FDA)-approved phase 2 basket trial has three highlights: (1) PRL3, an intracellular oncotarget that is highly (∼80.6%) expressed in multiple cancers; (2) PRL3-zumab, the first-in-class humanized antibody (immunoglobulin G1 [IgG1]) with high affinity to PRL3 (Kd = 7.57 pM); and (3) proof of concept: targeting intracellular oncoprotein with antibody-based therapy. A full analysis set (FAS, 51 patients received ≥1 dose) is used for pharmacokinetic and safety studies. Out of FAS, 20 patients are eligible to constitute the efficacy evaluable set (EES). To circumvent the heterogeneities from different individuals/cancers, we propose single evaluable patient single cohort (SEPSC) and apply comparison using double stringent/rigorous controls with (1) historical progression-free survival (PFS) and (2) prior lines’ PFS within the same patients. PRL3-zumab shows longer PFS than prior line(s) of anti-PD-(L)1 therapies. PRL3-zumab demonstrates excellent safety and clear clinical benefits in late-stage IV solid cancer patients. This trial is registered at ClinicalTrials.gov as NCT04452955.
Keywords: PRL3; intracellular oncoprotein; PRL3-zumab; phase 2 basket clinical trial; full analysis set,; FAS set; efficacy evaluable set,; EES set; single evaluable patient single cohort,; SEPSC; PFS; historical data; intra-patient comparison
Graphical abstract

Highlights
-
•
PRL3 is an intracellular PAN-oncotarget that can be externalized under tumor stresses
-
•
PRL3-zumab, a humanized antibody, binds to externalized PRL3 for cancer immunotherapy
-
•
PRL3-zumab demonstrates its excellent safety: no drug-related >grade 2 adverse events
-
•
PRL3-zumab shows its efficacies by providing longer PFS compared to historical data
Park et al. report the results of a FDA-approved phase 2 basket trial in advanced solid tumors for PRL3-zumab, a humanized antibody drug for cancer immunotherapy. PRL3-zumab shows its excellent drug safety and provides longer progression-free survival than historical data and prior lines of anti-PD-(L)1 therapies.
Introduction
Antibody-based cancer therapies have demonstrated better specificity and improved efficacy over standard chemotherapeutic regimens.1 However, antibody-based approach has been focused on a limited number of tumor-specific antigens at the cell surface. For the past decades, we had explored and demonstrated that antibody-based therapy also could be used to target tumor-associated intracellular targets, as an alternative to chemotherapy. In 1998, we identified the phosphatase of regenerating liver 3 (PRL3), an intracellular protein that belongs to the tyrosine phosphatase superfamily.2 In 2001, the Vogelstein group showed that PRL3 was overexpressed in metastatic liver lesions compared to corresponding primary colorectal tumors or normal colon epithelium.3 PRL3 is subsequently demonstrated to be associated with most cancer types, thus serving as a PAN-oncotarget.4 Conventionally, small chemical inhibitors are used to target intracellular PRL3 phosphatase because intracellular compartments are presumed to be inaccessible to large antibody molecules. However, regardless of how specific these intracellular antigens are, small chemical inhibitors can still cause non-specific off-target effects due to their poor specificity to these targets.
Since 2008, as an alternative to chemotherapy, our group has been working on the concept of targeting intracellular oncoproteins with antibody therapy, which has been published in a series of papers with various animal models.5,6,7,8,9 The animal models have reproducibly demonstrated the anti-tumorigenic property of PRL3 antibody against tumors that overexpressed intracellular PRL3 oncoprotein. Mechanistically, we revealed that intracellular PRL3 can be externalized on the cancer cell surface in tumor microenvironment when they were freshly isolated from in vivo tumor. The externalization or “inside-out” event of PRL3 was barely detectable (∼1.1%) in cultured cells but highly (64.9%) detectable in most ex vivo-dissociated single tumor cells.8 The externalization of PRL3 could be triggered by multiple factors in acidic tumor microenvironment,10 such as inflammation induced by host immune system, apoptosis, necrosis, or PRL3 presented on outer exosomal membranes. The antibody could then specifically bind to the externalized PRL3 at the surface of cancer cells to recruit host immune cells in a manner consistent with antibody-dependent cell-mediated cytotoxicity or antibody-dependent cellular phagocytosis. PRL3-zumab requires an intact Fc region and host FcγII/III receptor to engage B cells (but not T cells), natural killer (NK) cells, and macrophages to elicit anti-tumor activity against PRL3-expressing tumor masses.10,11
So far, we have not only demonstrated that PRL3 mouse antibody,5,6,11 PRL3 chimeric antibody,12 and PRL3-zumab7,8 could specifically block tumors that overexpress intracellular PRL3 oncoprotein but also demonstrated a similar phenomenon using antibodies against other intracellular oncoproteins as a general concept.6
Antibody targeting intracellular oncoproteins is a nascent area of immunotherapy.6 The implications of this concept were commended and elaborated in a perspective article titled “Hidden Immunotherapy Targets Challenge Dogma” by the late Professor Soldano Ferrone, a pioneering cancer immunologist.13 Our unprecedented translational cancer research was described in Cell Review as the leading edge in cancer immunotherapy and has triggered the thought that the potential of antibodies targeting intracellular antigens could have been underestimated.14 Targeting intracellular antigens would profoundly broaden antibody immunotherapy to include tumor-specific mutated intracellular proteins and other intracellular mediators of cell survival and proliferation. Recently, the PRL3-zumab paradigm has been further highlighted in Nature Review Drug Discovery review article titled “Targeting protein phosphatases in cancer immunotherapy and autoimmune disorders.”15
PRL3 protein was found to be overexpressed in 80.6% of 151 patients’ fresh-frozen tumor samples across 11 common cancers such as the liver, lung, colorectal, breast, gastric, thyroid, pancreatic, kidney, acute myeloid leukemia (AML), bladder, and prostate, but not in patient-matched normal tissues.8 In addition to its clinical relevance in diverse types of cancer, the key role of PRL3 has been well established in mediating cancer signaling and functions, including but not limited to proliferation, metastasis, and angiogenesis.4,16
PRL3-zumab phase I clinical trial was completed in National University Hospital Singapore in 2018 and demonstrated excellent drug safety profile.17 Multi-national phase 2 trials have been conducted in the US (NCT04452955), Singapore (NCT04118114), China (CTR20211180), and Malaysia (phase 2/3). To date, PRL3-zumab has not only shown its excellent safety profile but also significantly extended progression-free survival (PFS) and several partial responses in aggressive cancer types in ongoing trials. When late-stage IV cancer patients have no other therapeutic options, PRL3-zumab could still show its clinical benefits as the last option. Herein, we summarize and report our results of the Food and Drug Administration (FDA)-approved phase 2 clinical trial in the USA.
Results
Baseline characteristics and patient disposition
The patients of this PRL3-zumab phase 2 basket trial were recruited from 5 hospitals (study sites) in the US between December 2020 to March 2022 during the period of COVID-19 pandemic. 51 patients who received ≥1 dose (6 mg/kg) of PRL3-zumab were considered as a full analysis set (FAS) for safety and pharmacokinetic (PK) studies. Characteristics of patients at baseline are shown in Table S1. The median age of recruited patient was 65 (40–79), and male (47%) and female (53%) were almost equally distributed. Races were mostly white (62.7%) followed by Asian (15.7%), Hispanic or Latino (11.8%), and African American (9.8%). As this is a basket trial, multiple solid cancers were recruited with most common tumor type represented as colon and pancreatic tumor (19.6% each) followed by lung (11.8%); ovarian/fallopian tube; uterus/cervix; gall bladder/bile duct tumor (7.8% each); liver (5.9%); gastric and breast (3.9% each); and cecal, rectal, anal, kidney, nasopharyngeal, and sarcoma (2% each). Eastern Cooperative Oncology Group (ECOG) performance status scale was 72.5% (ECOG-1) and 27.5% (ECOG-0) of patients at the time of screening. It is challenging to reveal drug efficacies in overwhelming late-stage IV patients whose immune systems had been exhausted. Therefore, we first stratify the FAS set (51 total patients) into 2 groups: (1) evaluable patients who met at least the protocol inclusion criteria #5 with 6 months survival to receive ≥4 doses of study drug and ≥1 post-baseline computed tomography (CT) scans as efficacy evaluable set (EES) and (2) unevaluable patients, who did not meet the protocol inclusion criteria #5 with short survival, receiving <4 dose of study drug and no post-baseline CT scans. Secondly, since this is a basket trial with multiple cancer types, we proposed each evaluable patient as single evaluable patient single cohort (SEPSC) to recognize each patient as individual cohort. Thirdly, to avoid the heterogeneity of patients with different tumor types and different medical history, the drug efficacy of each SEPSC patient was compared with 2 stringent/rigorous controls: (1) compared with relevant cancer type of historical PFS as an external control and (2) compared with PFS of prior treatment lines in the same patients (intra-patient) as an internal control (Figure 1).
Figure 1.
A unique clinical design to study drug efficacy in a small basket trial patients with different cancer types/medical history
Step 1: 51 FAS patients were stratified into evaluable (EES), patients who received 4 doses with a post-baseline CT scan to compare with the baseline CT scan on tumor sizes, and unevaluable patients who did not meet EES criteria. Step 2: to avoid the heterogeneity of patient population, each patient in EES was considered as single evaluable patient single cohort (SEPSC). Step 3: to apply double stringent controls, (1) compatible historical data were used as an external control, and (2) prior therapy (intra-patient comparison) was used as an internal control since each patient suffered from different tumor types with different medical history. See also Table S1.
Primary endpoint
PFS
PRL3 is highly (80.6%) overexpressed in a broad range of cancer types.8 Since most of the patients were at late lines of stage IV, we set the clinical benefits of the drug treatment with PFS as the primary efficacy endpoint in the EES, which includes different cancer types with a life expectancy of ≥6 months, having a “runway for immunotherapy” to respond to PRL3-zumab to elicit anti-tumor responses. Median PFS values for each cancer types were as follows: gastric, 7.78 (2.23–13.33) months; colorectal, 3.74 (1.87–4.67; 95% confidence interval [CI], 2.09–4.24) months; lung cancer, 3.77 (1.87–5.63; 95% CI, 2.08–4.88) months; anal cancer, 10 months; cervical cancer, 5.6 months; ovarian cancer, 3.9 months; renal cancer, 2.6 months; breast, fallopian tube, and pancreas cancer, 1.87 months each. Rate of PFS was analyzed from PFS-2 to PFS-12 and mentioned in Table 1.
Table 1.
PFS in efficacy evaluation set
| Patients (n = 20) | Median PFS (months) | Rate of PFS |
|||||
|---|---|---|---|---|---|---|---|
| PFS-2 | PFS-4 | PFS-6 | PFS-8 | PFS-10 | PFS-12 | ||
| Gastric cancer (2) | 7.78 (2.23–13.33) | 100% | 50% | 50% | 50% | 50% | 50% |
| Anal cancer (1) | 10 | 100% | 100% | 100% | 100% | 100% | 0% |
| Colorectal cancer (5) | 3.7 (1.87–4.67) | 60% | 20% | 0% | – | – | – |
| Lung cancer (5) | 3.77 (1.87–5.63) | 60% | 60% | 0% | – | – | – |
| Cervical cancer (1) | 5.6 | 100% | 100% | 0% | – | – | – |
| Ovarian cancer (1) | 3.9 | 100% | 100% | 0% | – | – | – |
| Renal (1) | 2.6 | 100% | 0% | – | – | – | |
| Breast (2) | 1.87 | 0% | – | – | – | – | – |
| Fallopian tube cancer (1) | 1.87 | 0% | – | – | – | – | – |
| Pancreas (1) | 1.87 | 0% | – | – | – | – | – |
Rate of PSF-2, 4, 6, 8, 10, and 12 = rate of patient who achieved progression-free survival of 2, 4, 6, 8, 10, and 12 months.
PRL3-zumab showed significantly longer PFS than historical clinical data of respective cancer types
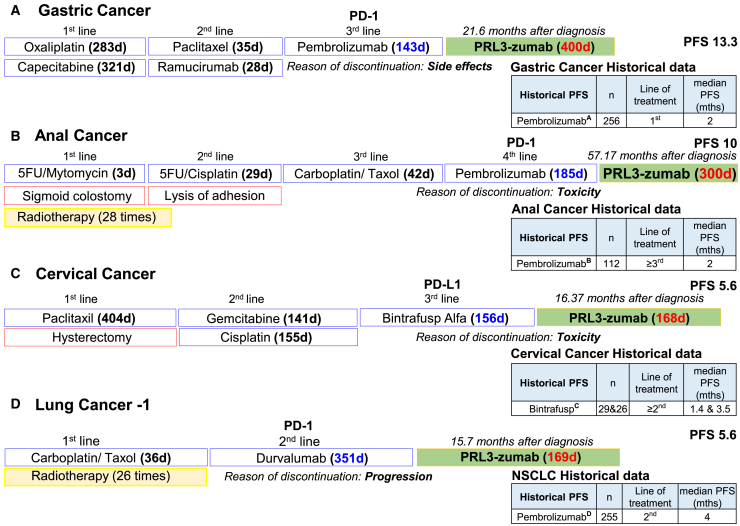
To gain a thorough understanding of each patient’s response, PRL3-zumab therapeutic efficacy for each SEPSC was analyzed using an external control arm to compare with historical clinical data at the compatible or even earlier lines (2nd or 3rd) in the relevant tumor type although most of the patients were 4th or 5th line of treatment for PRL3-zumab therapy. Among the 20 SEPSCs, 9 patients (A–I) could be compared with the historical PFS of whom 8 patients (A–H) showed longer PFS than the historical data of the relevant tumor types (Figure 2). Among the 9 patients, patient A with late-stage IV gastric cancer received PRL3-zumab as 4th line, and the drug achieved the longest PFS of 13.33 months (400 days) with significant improvement in quality of life. This case was reported on the webpage of Providence St. Jude Medical Center.18
Figure 2.
Swimmer plot of PFS in EES patients
Historical PFS as an external control arm. PRL3-zumab PFS of 9 patients (A–I) (blue) were compared with historical references (orange) of respective cancer type. The red triangle indicates stable disease (SD) at designated time point for disease assessment. Historical reference: for patient A, pembrolizumab in gastric cancer-KEYNOTE-05919 (NCT02335411); for patient B, pembrolizumab in anal cancer-KEYNOTE-15820 (NCT02628067); for patient C, tisotumab/vedotin in cervical cancer21 (NTC03438396); for patients D–E and I, pembrolizumab in NSCLC- KEYNOTE-1022 (NCT01905657); for patient F–H, chemotherapy in colon cancer and phase 3 RECOURSE trial23 (NCT01607957). See also Figure S1.
Historical PFS data for gastric cancer therapies taken from KEYNOTE-059 (NCT02335411) for 3rd line standard of care (SOC) and from CHECKMATE-649 study24 with chemotherapy in combination with anti-PD-1 (nivolumab) have a median PFS of 7.7 months, while chemotherapy in combination with anti-HER2 (trastuzumab) has a median PFS of 6.7 months (ToGA study)25 (Figure S1). In addition, patient A’s tumor target lesion was reduced by ≤20% from baseline (6 post-baseline CT scans in total) (Figure S2).
In Figure 2, patient B, an anal cancer patient, received PRL3-zumab as 4th line treatment and showed PFS = 10.0 months (300 days) that is much longer than the historical data (median PFS = 2 months) taken from the phase 2 KEYNOTE-158 (NCT02628067), pembrolizumab as ≥2nd line treatment in stage IV anal cancer. Patient C, the cervical cancer patient, had PFS = 5.60 months (168 days) with PRL3-zumab as 4th line therapy. As a historical reference, tisotumab/vedotin study (NCT03438396) demonstrated a median PFS of 4.2 months as 2nd/3rd line treatment. Patients D and E, the two lung cancer patients, received PRL3-zumab, respectively, as 4th line and 7th line therapy. The PFS was 5.63 (169 days) and 4.20 months (126 days), respectively. There is no compatible 4th and 7th lines of historical data available references. Therefore, we used earlier lines of historical reference, the phase 2/3 KEYNOTE-010 study (NCT01905657), on non-small cell lung carcinoma (NSCLC) and found that 2nd line pembrolizumab treatment had a median PFS of 5.2 months for PD-L1-high tumors (≥50% expression) and 4.0 months for all-comers. PRL3-zumab at 4th and 7th lines of treatments still appeared to be better than the PFSs for some cancer types at 2nd line of treatments.
In addition, there were also two colon cancer patients (patients F and G) and one rectal cancer patient (patient H). For patient F, whose PFS was 4.67 months (140 days), PRL3-zumab was used as 4th line. For patient G, PRL3-zumab was administered as 5th line therapy after progression on chemotherapy, and PFS was 3.7 months (111 days). Patient H, a rectal cancer patient, received PRL3-zumab as 4th line therapy, and PFS was 3.77 months (113 days). As a historical reference, the phase 3 RECOURSE trial of ≥3rd trifluridine/tipiracil (NCT01607957) showed a median PFS of 2.0 months. Compared to the current SOC treatments, PRL3-zumab treatment led to a longer PFS at late lines of stage IV patients, better than earlier lines of SOCs. However, it should also be noted that the recent phase 3 SUNLIGHT trial of trifluridine/tipiracil in combination with bevacizumab (NCT04737187) demonstrated a median PFS of 5.6 months, though this regimen has yet to be approved. Patient I with lung cancer (at 2nd line) had PFS of 3.77 months (113 days), shorter than historical reference. There are no compatible late lines of historical reference for patients J and K; hence, no comparison can be made.
Based on A’Hern design26 α = 0.05, 1-β = 0.8, PRL3-zumab treatment resulted in significantly longer PFS (in blue bars) in 8/9 SEPSCs (patients A–H) in this study, as compared to historical PFS references (in orange bars) of respective cancer types in comparable patients (external control arm). This included patients with gastric, anal, cervical, lung, colon, and rectal cancers, to clearly demonstrate the drug efficacy of SEPSC patients with PRL3-zumab therapy.
Secondary endpoint
Clinical benefit rate and best overall response of PRL3-zumab therapy
Evaluation of tumor response was performed as per response evaluation criteria in solid tumors 1.1 (RECIST 1.1) in the EES set. Clinical benefit rate (CBR) (partial response, PR, or stable disease, SD) at 8, 16, and 24 weeks was 55%, 25%, and 10%, respectively (Table 2). The best overall response was SD, detected in 11 out of 20 (55%) at 8 weeks; 5 out of 20 at 16 weeks; 2 out of 20 at 24, 32, and 40 weeks; and 1 out of 20 at 48 and 56 weeks of tumor assessment in EES (Table 2). One gastric cancer patient (A) demonstrated SD from 8 to 56 weeks (400 days), and one anal cancer patient achieved SD until 40 weeks (Figures 2 and S2). Progressive disease (PD) was detected in 9 patients at 8 weeks. As most of the patients were at the last line of stage IV, objective response rate (ORR, PR and complete response) was not achieved. We would expect to have better efficacy in earlier stage IV patients with fewer prior lines.
Table 2.
Clinical benefit rate and best overall response in 20 evaluable patients
| n = 20 | Week 8 | Week 16 | Week 24 | Week 32 | Week 40 | Week 48 | Week 56 |
|---|---|---|---|---|---|---|---|
| Clinical benefit rate, overall response, n (%) RECIST v.1.1 | |||||||
| Stable disease | 11 | 5 | 2 | 2 | 2 | 1 | 1 |
| Progressive disease | 9 | 15 | 18 | 18 | 18 | 19 | 19 |
| Clinical benefit rate (CBR) | 55% | 25% | 10% | 10% | 10% | 5% | 5% |
| Best overall response (n=20) | |||||||
| Gastric cancer, n=2 | 1 SD | 1 SD | 1 SD | 1 SD | 1 SD | 1 SD | 1 SD |
| Anal cancer, n=1 | 1 SD | 1 SD | 1 SD | 1 SD | 1 SD | 0 | 0 |
| Colorectal cancer, n=5 | 3 SD | 1 SD | 0 | 0 | 0 | 0 | 0 |
| Lung cancer, n=5 | 3 SD | 1 SD | 0 | 0 | 0 | 0 | 0 |
| Cervical cancer, n=1 | 1 SD | 1 SD | 0 | 0 | 0 | 0 | 0 |
| Ovarian cancer, n=1 | 1 SD | 0 | 0 | 0 | 0 | 0 | 0 |
| Renal cancer, n=1 | 1 SD | 0 | 0 | 0 | 0 | 0 | 0 |
| Breast cancer, n=2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fallopian tube cancer, n=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pancreas cancer, n=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
In 3 out of 4 patients, PRL3-zumab showed superior efficacy to prior lines of PD-1/PD-L1 immunotherapy
In this trial, there are 4 SEPSCs who had terminated PD-1/PD-L1 immunotherapy and subsequently received PRL3-zumab therapy (Figure 3). Patients A, B, and C discontinued PD-1/PD-L1 immunotherapies due to toxicity/side effect at 4.8, 6.2, and 5.2 months, respectively, with gap (63, 42, and 104 days, respectively) before receiving PRL3-zumab. This “head to tail” intra-patients’ comparison (within the same patients) is to bypass patient heterogeneity in different cancer types with different medical history. PRL3-zumab treatment resulted in a longer PFS than prior lines of PD-1/PD-L1 therapies. Statistical consideration was based on A’Hern design,26 α = 0.05, 1 - β = 0.8, P0 = 20%, and P1 = 85%. PRL3-zumab demonstrated a significantly longer PFS in the same patients (A, B, and C) at 13.33 (400 days), 10 (300 days), and 5.6 months (168 days) with the exception of patient D, suggesting that PRL3-zumab could serve as a rescue therapy in cancer patients who discontinue PD-1/PD-L1 immunotherapies due to toxicities/side effects. In general, we have observed a trend that PRL3-zumab could outperform PD-1/PD-L1 immunotherapy in extending PFS for patients who are refractory to or unsuitable for PD-1/PD-L1 immunotherapy (Figure 3).
Figure 3.
Intra-patient comparison as an internal control arm
To avoid the heterogeneity of patient population since each patient suffered from different tumors with different natural histories. PRL3-zumab showed superior efficacy compared to prior lines of PD-1/PD-L1 immunotherapies in patients A–C. The diagram shows prior line of therapy and duration, duration of PRL3-zumab therapy, and historical data for related disease. Historical references: A, KEYNOTE-5919 (NCT02335411); B, KEYNOTE-15820 (NCT02628067); C, a trial of tisotumab/vedotin in cervical cancer21 (NCT03438396); D, KEYNOTE-1022 (NCT01905657). d, days.
Overall survival
The median overall survival (OS) for gastric cancer (n = 2) is 12.93 (9.73–16.13) months, that for colorectal cancer (n = 5) is 7.87 (7.43–12; 95% CI, 6.35–10.19) months, and that for lung cancer is 14.85 (12.13–18.63; 95% CI, 12.57–17.13) months. Median OS of anal cancer (n = 1) is 13.8 months, that for cervical cancer (n = 1) is 13.03 months, that for ovarian cancer (n = 1) is 6 months, that for breast cancer (n = 2) is 9.15 months (8.73–9.57), that for renal cancer (n = 1) is 15.4 months, that for fallopian tube cancer (n = 1) is 6 months, and that for pancreatic cancer is 6.37 months. 2 lung cancer patients, 1 renal cancer patient, 2 breast cancer patients, and 1 fallopian cancer patient are still alive at the time of the last survival monitoring (Table 3).
Table 3.
Overall survival in efficacy evaluation set
| Cancer type | OS | Median OS | 95% CIa |
|---|---|---|---|
| Gastric cancer | 16.13 | 12.93 (9.73–16.13) | – |
| 9.73 | |||
| Anal cancer | 13.80 | 13.80 | – |
| Colorectal caner | 12.00 | 7.87 (7.43–12) | 6.35–10.19 |
| 7.87 | |||
| 7.87 | |||
| 6.20 | |||
| 7.43 | |||
| Lung cancer | 18.63b | 14.85 (12.13–18.63) | 17.13–12.57 |
| 13.87 | |||
| 16.30 | |||
| 13.33 | |||
| 12.13b | |||
| Cervical cancer | 13.03 | 13.03 | – |
| Ovarian cancer | 6.00 | 6.00 | – |
| Renal cancer | 15.40b | 15.40 | – |
| Breast cancer | 8.73b | 9.15 (8.73–9.57) | – |
| 9.57b | |||
| Fallopian tube cancer | 6.00b | 6.00 | – |
| Pancreas cancer | 6.37 | 6.37 | – |
CI, confidence interval.
Patient survival status is recorded as “alive” at the last monitoring.
Target lesion size
Changes in total diameter of target lesions in each patient of EES are presented as a waterfall plot (Figure S2). PRL3-zumab can achieve the best overall response of SD in target lesion from 14/20 (70%) of patients in EES on the first tumor evaluation time point, 8 weeks.
PRL3-zumab PKs
In this trial, sparse PK samples were collected from 41 patients. The collected time points varied depending on the duration of the trial in each patient. The mean concentration-time curve of sparse PK samples is mentioned in Figure S3A. All data points represent mean ± SD. Noncompartmental model analysis was done based on the PK sample of the first dose. Mean plasma half-life (T1/2) was 8.5 ± 4.7 days, volume of distribution (Vd) was 3.5 ± 0.98 L, area under concentration (AUC) was 1,501.8 ± 757.4 μg/mL/day, and maximum concentration at steady state (Css Max) was 107.27 ± 54.1 μg/mL. Minimum concentration at steady state (Css Min) was 40 μg/mL, as interpreted from the concentration-time curve. T1/2 and Vd are comparable to those of phase 1 clinical trial.17 We proposed the dose interval of 12 ± 2 days at 6 mg/kg.
The extensive PK samples were collected from two patients; sample collection was performed up to cycle 6 for one patient and up to cycle 2 for the other patient. The concentration-time curve showed that the plasma drug concentration of both patients was comparable (Figure S3B). Noncompartmental model analysis was performed as single-dose kinetics on PK samples of 1st dose (C1D1) (C1D1 pre-dose, end of infusion, and 2 h and 6 h post-infusion, C1D2, C1D6, C1D10, C1D15 pre-dose), 2nd dose (C1D15 pre-dose, C1D15 post-dose, and C2D1 pre-dose) in both patients, and 6th dose (C1D15) (C3D15 pre-dose, end of infusion, 2 h, 6 h, C3D1, C3D20, and C3D24) in one patient. PK parameters were mentioned as mean ± SD values. Mean plasma half-life (T1/2) was 22.02 ± 15.38 days, volume of distribution (Vd) was 5.5 ± 2.07 L, AUC was 2,962.94 ± 1,522.82 μg/mL/day, and maximum concentration at steady state (Css Max) was 123.46 ± 63.45 μg/mL. Minimum concentration at steady state (Css Min) was 50 μg/mL, as interpreted from the concentration-time curve. Longer T1/2 was detected in multiple-dose administration. Css Min of both sparse and extensive PK were comparable with our animal study Css Min (50 μg/mL).
We concluded that a 10- to14-day per dose interval is enough to maintain Css Min at 50 μg/mL. Since most of the patients are at late stages with rapidly deteriorating health, to achieve the required Css earlier to timely control heavy tumor burdens, the dose interval for the initial four doses can be shortened to a 1-week interval, followed by subsequent 2-weekly/dose.
Safety
PRL3-zumab showed excellent safety in the FAS set (51 patients in total). 348 treatment-emergent adverse events (TEAEs) (1–4 grade adverse events [AEs]) from 21 systems/organs were reported. 290 (83.3%) were grade ≤2. 0.6% out of them were “possibly related,” and 0.9% were “related” to the study drug. 58 TEAEs (16.7%) were grade ≥3, none of which was related to the study drug. The percentages of AEs in each system and their relationship to the study drug are highlighted in Table 4. Four patients discontinued the study due to AE of hyponatremia, dyspnea, worsening lymphedema, and worsening anemia, which were “unrelated” to the study drug. A total of 43 severe adverse events (SAEs) were reported in 21 patients. None was suspected unexpected serious adverse reaction (SUSARs), and none was related to the study drug (Table S2). Patients died shortly after the start of the trial, which was due to their deteriorating terminal disease conditions that were “unrelated” to the study drug.
Table 4.
Treatment-emergent adverse events in US phase 2 trial patients
| Relationship to PRL3-zumab | Unrelateda |
Possibly relateda |
Relateda |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ≥4 | 1 | 2 | 3 | ≥4 | 1 | 2 | 3 | ≥4 | |
| Any TEAEb | 127 | 105 | 55 | 3 | 25 | 13 | 0 | 0 | 14 | 6 | 0 | 0 |
| Any serious TEAE | 0 | 2 | 36 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Action taken because of TEAE | ||||||||||||
| Dose interruption | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dose reduction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Treatment discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TEAE in each system | ||||||||||||
| Nervous system disorders | 9 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 7 | 11 | 2 | 1 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 |
| Cardiac disorders | 3 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psychiatric disorders | 4 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | 19 | 22 | 14 | 0 | 10 | 1 | 0 | 0 | 4 | 0 | 0 | 0 |
| Hepatobiliary disorders | 0 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Musculoskeletal/connective tissue disorders | 13 | 10 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Renal and urinary disorders | 2 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Eye disorders | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reproductive system and breast disorders | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| General/administration site disorders | 15 | 9 | 3 | 0 | 7 | 4 | 0 | 0 | 0 | 1 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 4 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Blood and lymphatic system disorders | 5 | 5 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vascular disorders | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Neoplasms benign, malignant, and unspecified | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metabolism and nutrition disorders | 17 | 20 | 2 | 0 | 1 | 3 | 0 | 0 | 2 | 0 | 0 | 0 |
| Infections and infestations | 2 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Investigations | 13 | 5 | 6 | 0 | 3 | 2 | 0 | 0 | 0 | 1 | 0 | 0 |
| Injury, poisoning, and procedural complications | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ear and labyrinth disorders | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Endocrine disorders | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
“Unrelated, possibly related, related” indicate relationship to study drug.
TEAE, treatment-emergent adverse event.
Discussion
The PRL3-zumab paradigm represents an unprecedented cancer immunotherapy of targeting intracellular oncoprotein with antibody therapy. PRL3-zumab showed its excellent drug safety profile in the first-in-man phase 1 trial with 27 end-stage cancer patients (23 solid tumors and 4 AML patients) in Singapore.17 To further assess the drug’s efficacy and safety in patients, we then proceeded to multi-national phase 2 trials approved by the Health Sciences Authority in Singapore (NCT04118114), FDA in the US (NCT04452955), National Medical Products Administration in China (CTR20211180) and phase 2/3 trial approved by National Pharmaceutical Regulatory Agency in Malaysia. So far, the drug has been administered in ∼ 200 patients performed in multi-national clinical trials, showing only a few AEs of grade 2.
In this FDA-approved phase 2 basket oncology trial, PRL3-zumab further showed its excellent drug safety in all 51 patients (FAS set). There were no drug-related infusion reactions, no drug-related AEs 3–4, no drug-related dose limiting toxicities (DLTs), no drug-related SUSAR, no drug-related SAEs, and no drug-related deaths since PRL3 is a tumor-specific target and PRL3 expression in normal tissues is undetectable. PRL3-zumab also showed similar T1/2 and Vd as phase 1 study for noncompartmental analysis.17 The PK parameters showed that the required Css was achieved after 2nd dose of PRL3-zumab administrations in recent dose regimen, the 2-week (+/− 2 days) dose interval.
Due to the cancer patients being at the advanced late-stage IV, most patients could only participate in the trial for a short duration, which gave very limited time for PRL3-zumab immunotherapy to take effect because of the exhausted immune system by prior lines of treatment. It is very challenging to study PRL3-zumab drug efficacy using predominantly end-stage cancer patients with rapidly deteriorating health conditions.
Nevertheless, PRL3-zumab still firmly demonstrated its drug efficacy (as per RECIST v.1.1) in this unique clinical trial design with an unconventional methodology of data analysis: First, we stratified unevaluable patients (received <4 doses with no post-baseline CT scan on tumor sized) from evaluable patients (received ≥4 doses and with ≥1 post-baseline CT scan for tumor sizes, ≥6 months survival). Second, as this is a basket trial that includes multiple cancer types, we propose the concept of SEPSC. Third, since PRL3-zumab treatment was administered at the late lines of stage IV cancer patients, PFS (but not ORR) and CBR were then set as the primary endpoints. Fourth, due to a small patient sample size, we applied stringent/rigorous double controls to demonstrate drug efficacy with high confidence.
Historical PFS data of cancer drugs in clinical trials of respective cancer type as an external control arm (control 1): among 20 SEPSCs, 55% (11 out of 20) were SDs (Table 2). 72.8% (8 out of 11) of SDs demonstrated significantly (R.P.A′ HERN statistics) longer PFS compared to relevant historical PFS. PRL3-zumab therapy achieved significantly longer PFSs compared with available similar lines or earlier lines of historical PFS data from respective cancer types (Figure 2). Regulatory agencies now accept single-arm trials with external historical controls, particularly common in oncology, to assess promising treatments for rare or unmet indications.27 Intra-patients’ comparison (head to tail) as an internal control arm to avoid heterogeneity of patients suffering from different cancer types with different medical histories (control 2): 75% (3 out of 4) SEPSCs in which PRL3-zumab was the next-in-line treatment after the failure of anti-PD-(L)1 immunotherapy due to drug toxicity/side effects. We evaluated the therapeutic efficacy of PRL3-zumab versus prior SOC and anti-PD-(L)1 immunotherapy in the same patients (Figure 3) whose prior lines can serve as a benchmark for an investigational drug’s efficacy and have the advantage of avoiding inter-patient heterogeneity with different tumor type, metastatic status, and medical history. In summary, PRL3-zumab has shown clinical benefits with longer PFS compared to historical data or prior lines of SOC and anti-PD-(L)1 immunotherapy in late-stage IV cancer patients.
Entrectinib is a tropomyosin receptor kinase inhibitor and was approved for the treatment of neurotrophic tyrosine receptor kinase (NTRK) fusion-positive solid tumors based on intra-patient comparison for efficacy assessment in a single-arm trial.28 Intra-patients’ comparison has the advantage of a quick turnaround time to see drug efficacy compared to prior lines of treatment. Intra-patient comparisons are more stringent/rigorous controls than conventional head-to-head (side by side) control or randomized controlled trials (RCTs). RCTs, for instance, are not feasible for molecular targeted agents in rare indications because of small patient numbers and slow patients’ recruitment28 to give further insight into drug efficacy, which will consequently delay the potential clinical benefits to patients with the urgent medical needs.
As PRL3 is a PAN-oncotarget overexpressed at high frequency (80.6%) in multiple cancer types, PRL3-zumab immunotherapy could potentially bring clinical benefit to patients suffering from many different cancer types. PRL3-zumab adds months or years (of PFS) with a good quality of life to an incurable cancer stage IV patient’s life as compared to the same cancer type of historical PFS data from SOC treatments and has significantly longer PFS and better safety profile compared to the prior treatment lines. PRL3-zumab could serve as an alternative option for urgent unmet medical needs such as aggressive or rare stage IV cancer patients (lesser SOCs) with >6 months of life expectancy. PRL3-zumab could be a rescue therapy for cancer patients who are refractory to or unsuitable for PD-1 or PD-L1 immunotherapies.
Limitations of the study
-
(1)
The trial was conducted during the COVID-19 pandemic, which presented challenges in patient recruitment. As a result, overwhelming late-stage IV patients were recruited into this trial.
-
(2)
The drug did not have opportunity to demonstrate its efficacy in earlier lines of treatment for stage IV patients.
-
(3)
The EES dataset was analyzed with RECIST 1.1 (instead of I-RECIST), limiting the ability to accurately monitor the responses to immunotherapy.
-
(4)
Given the small number of patients across multiple cancer types, we proposed the SEPSC approach and implemented tougher double stringent controls: (1) historical PFS and (2) prior lines PFS within the same patients to validate drug efficacy.
Conclusion
The PRL3-zumab paradigm is the proof-of-concept human trial that antibodies can target intracellular antigens to elicit anti-tumor immunity based on the mechanism that intracellular oncoproteins can be externalized in tumor microenvironment.8 Further investigation into this unconventional immunotherapy could expand our repertoire of cancer therapy in the future.
Resource availability
Lead contact
Requests for further information, resources, and reagents should be directed to and will be fulfilled by the lead contact, Qi Zeng (mcbzengq@imcb.a-star.edu.sg).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. This paper does not report any original code. Any additional information required to reanalyze the data reported in this paper is available from lead contact upon request.
Acknowledgments
We thank the patients and families who made this trial possible. We thank the investigators and their staffs from all 5 study sites for their participation in this FDA-approved phase 2 clinical trial. This study was funded by Intra-ImmuSG Pte Ltd, an A∗STAR (Agency for Science, Technology and Research, Singapore) spin-off company. We are very grateful to Professor Wanjin Hong for his kind support and advice on PRL3-zumab development and his critical reading of this manuscript.
Author contributions
Conceptualization, Q.Z. and M.T.; methodology, Q.Z. and M.T.; investigation, D.J.P., V.K.C., and B.V.; project administration, D.J.P., V.K.C., B.V., and B.S.; formal analysis, Q.Z., M.T., D.J.P., K.H.A., P.L.C., K.Y.K., J.L., K.Z., W.H.Z., and M.C.H.N.; writing – original draft, M.T., D.J.P., and Q.Z.; writing – reviewing and editing, Q.Z., D.J.P., M.T., and K.H.A.; supervision, Q.Z. and M.T.
Declaration of interests
The PRL3 antibody was patented under patent number US11155636B2 by the Agency for Science, Technology and Research (A∗STAR) in Singapore. Q.Z. is the founder of Intra-immuSG Pte Ltd, a spin-off company from A∗STAR that has been granted licensing rights for the PRL3-zumab IP portfolio.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-PRL3-zumab antibody | Abgent/ Wuxi Apptec | N/A |
| Biological samples | ||
| Human plasma samples | Participating study sites | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Humanized PRL3-GST | Research Lab IMCB, A∗STAR | N/A |
| Software and algorithms | ||
| Discovery Workbench 4.0 | Meso Scale Discovery | https://www.mesoscale.com/ |
| Watson LIMS | Thermo Fisher Scientific | https://www.thermofisher.com |
| PK Solver | Zhang et al.29 | https://www.sciencedirect.com/science |
Experimental model and study participant details
Study design and participants
PRL3-zumab, multicenter, open label, single dose level Phase II basket clinical trial was approved by FDA in Feb 2020 and conducted in 5 study sites/ hospitals in the US between December 2020 to March 2022.
The inclusion criteria included male or female patient aged ≥ 18 years-old with unresectable or metastatic solid tumors, willing to provide written informed consent, histopathological diagnosis and metastatic status cancer at study entry, must have received at least 1-3 prior line of systemic therapy for metastatic disease, life expectancy of ≥6 months, Eastern Cooperative Oncology Group (ECORG) performance status (PS) score of less than 2, adequate hematological and organ function (Absolute neutrophil count ≥ 1.0 x 109/L, Platelet count ≥ 75 x 109/L, hemoglobin ≥ 9 g/dL, total bilirubin ≤ 1.5x ULN, aspartate aminotransferase and alanine aminotransferase ≤ 2.5 x upper limit of normal (ULN) (≤ 5 x ULN in the presence of liver metastases), creatinine < 1.5x ULN, creatine clearance (according to Cockcroft-Gault Equation or by 24-hour urine collection) of > 60 mL/min at study entry, prothrombin time and activated partial thromboplastin time ≤ 1.5 x upper limit of normal (ULN) per institutional laboratory normal range, measurable disease by RECIST 1.1 and no evidence of active hepatitis B or C infection. Exclusion criteria were patients with known untreated or symptomatic central nervous system metastasis, female patient with pregnancy or lactation, patient with known human immunodeficiency infection, patient receiving systemic glucocorticoids or other immunosuppressive treatment, patient who experienced a severe hypersensitivity reaction to another monoclonal antibody, patient who received any systemic anti-cancer therapies within 3 weeks prior to study treatment, patient who undergone radiotherapy ≤4 weeks or limited field radiation for palliation ≤2 weeks prior to starting study treatment, patient who received >3 lines of prior systemic chemotherapy, patient who is unable to provide informed consent, patient who has history of another active cancer within 2 years, patient who received prior stem cell or bone marrow transplant, patient who received a live vaccine within 12 weeks and patient who is currently participating in a study on an investigational agent within 4 weeks.
The trial was approved by the Western-Copernicus Group (WCG) Institutional Review Board (IRB). The study was conducted in compliance with the principles of the Declaration of Helsinki and International Council for Harmonization Good Clinical Practice guidelines. All patients provided written informed consent prior to enrolment.
Study endpoints
As most cancer patients were at very terminal late-Stage IV in the trial, the primary efficacy endpoint was then set as Progression-Free Survival (PFS), defined as the time from the initiation of study treatment to the date of disease progression. Each patient’s PFS was compared with double stringent controls: 1) historical PFS references for related cancer types as external control arm; and 2) same patient’s prior lines of PFS as an internal control arm (intra-patient comparison). Secondary efficacy endpoints were Clinical Benefit Rate (CBR), Objective Response Rate (ORR) and Overall survival (OS). The PK parameters for PRL3-zumab after single and multiple dose administration include but not limit to Cmax (Maximum plasma PRL3-zumab concentration), Tmax (Time of Cmax), AUCinf: (Area under the concentration-time curve from pre-dose (time 0) extrapolated to infinite time) and t½ (Terminal elimination half-life) were analyzed. Safety end points included adverse events, serious adverse events and their relationship to the study drug. Safety was assessed by CTCAE version 5 criteria with adverse event (AE) and serious adverse event (SAE).
Method details
Study treatment and assessments
The study consists of a screening period (Day -21 to Day -1) where the first dose was considered as Cycle 1 Day 1 (C1D1). PRL3-zumab was administered intravenously every 2 weeks, 2 doses per Cycle until an end-of-treatment (EOT) patient progresses as per RECIST 1.1 or develops intolerable toxicity or withdraws consent. A safety follow-up visit was done at 14 ± 4 days after the last dose. PRL3-zumab (6.0 mg/kg) was administered by intravenous (IV) infusion.
All patients underwent baseline brain imaging (magnetic resonance imaging [MRI] with and without gadolinium or computed tomography [CT] with contrast), chest CT, abdomen, and pelvis imaging (CT with contrast) at the time of screening. The tumor evaluation was performed at baseline (within 28 days) before Cycle 1 Day 1 (C1D1) and for every 56 calendar days from Day 1 of the treatment, after every 4 doses, with contrast enhanced computed tomography (CT) scans. Response evaluation was performed according to response evaluation criteria in solid tumor version 1.1 (RECIST 1.1) and immune based response evaluation criteria in solid tumor (iRECIST). Safety was monitored from the first dose of PRL3-zumab until 14 days after last dose. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). Patients were followed for adverse events for at least 14 days after the last study dose.
Pharmacokinetics
Blood samples for Pharmacokinetic (PK) analysis in the sparse sampling group were collected on C1D1 (pre-dose and end of infusion), C1D15 (pre-dose), C2D1 (pre-dose and end of infusion), C3D1 (pre-dose, and endo of infusion), and end of treatment (EOT) and the extensive sampling group were collected on C1D1 (pre-dose, end of infusion, and 2 hrs, 6 hrs post-infusion), C1D2 (24 hours post-infusion), C1D6 (120 hours post-infusion), C1D10 (216 hours post-infusion), C1D15 (pre-dose), C2D1 (pre-dose), C2D15 (pre-dose), C3D1 (pre-dose), C3D15 (pre-dose, end of infusion, and 2 hours, and 6 hours post-infusion), C3D16 (24 hours post-infusion), C3D20 (120 hours post-infusion), C3D24 (216 hours post-infusion), C4D1 (pre-dose), C5D1 (pre-dose), C6D1(pre-dose), and end of treatment.
The plasma concentration of PRL3-zumab was analyzed by electrochemiluminescent method using MSD SECTOR S600 with an assay range of 20 to 2,560 ng/mL (with 10 ng/mL and 5,120 ng/mL as anchor points) for the quantitation of PRL3-zumab in human serum. Humanized PRL3-GST was used as captured reagent and rabbit polyclonal anti PRL3-zumab was used as detection reagent.
Raw data were captured using Discovery Workbench™, MSD 4.0 (Meso Scale Discovery, Gaithersburg, Maryland, U.S.A), and raw counts were reported with whole numbers. The concentrations calculated by Watson LIMS were reported as ng/mL with 3 significant figures. The figures listed for %RE and %CV will be rounded to 2 decimal places. Calculation of Pharmacokinetic parameters were done by PK solver software.29
Definition of endpoints
Progression free survival
The duration from the start of treatment (C1D1) to end of trial (EOT).
Clinical benefit rate
The percentage of patients who have achieved complete response (CR), partial response (PR) or stable disease (SD) overall and at 8 weeks, 16 weeks, and 24 weeks per RECIST 1.1 based on Investigator's assessment.
Overall response rate
The percentage of patients who have achieved complete response (CR) or partial response (PR) overall and at 8 weeks, 16 weeks, and 24 weeks per RECIST 1.1 based on Investigator assessment.
Overall survival
The duration from time of screening to time of death.
Quantification and statistical analysis
All patients who received ≥1 dose of PRL3-zumab was considered as Full Analysis Set (FAS) for safety study. Patients who received ≥4 doses of drug, with ≥1 post-baseline tumor assessment, ≥6 months survival, and no major protocol variation were considered as Efficacy evaluation set (EES) for drug efficacy study.
The analyses are exploratory. The median progression-free and overall survival were calculated with Clopper–Pearson 95% confidence interval. Rate of Progression Free Survival at 2 months, 4 months, 6 months, 8 months, 10 months and 12 months, Clinical Benefit Rate, Overall Survival Rate and Objective Response Rate were summarized as frequency and percentage.
Additional resources
The study is registered with ClinicalTrials.gov, NCT04452955.
Published: May 8, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2025.102120.
Contributor Information
Min Thura, Email: mthura@imcb.a-star.edu.sg.
Qi Zeng, Email: mcbzengq@imcb.a-star.edu.sg.
Supplemental information
References
- 1.Noguchi T., Kato T., Wang L., Maeda Y., Ikeda H., Sato E., Knuth A., Gnjatic S., Ritter G., Sakaguchi S., et al. Intracellular tumor-associated antigens represent effective targets for passive immunotherapy. Cancer Res. 2012;72:1672–1682. doi: 10.1158/0008-5472.CAN-11-3072. [DOI] [PubMed] [Google Scholar]
- 2.Zeng Q., Hong W., Tan Y.H. Mouse PRL-2 and PRL-3, two potentially prenylated protein tyrosine phosphatases homologous to PRL-1. Biochem. Biophys. Res. Commun. 1998;244:421–427. doi: 10.1006/bbrc.1998.8291. [DOI] [PubMed] [Google Scholar]
- 3.Saha S., Bardelli A., Buckhaults P., Velculescu V.E., Rago C., St Croix B., Romans K.E., Choti M.A., Lengauer C., Kinzler K.W., Vogelstein B. A phosphatase associated with metastasis of colorectal cancer. Science. 2001;294:1343–1346. doi: 10.1126/science.1065817. [DOI] [PubMed] [Google Scholar]
- 4.Chia P.L., Ang K.H., Thura M., Zeng Q. PRL3 as a therapeutic target for novel cancer immunotherapy in multiple cancer types. Theranostics. 2023;13:1876–1891. doi: 10.7150/thno.79265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo K., Tang J.P., Tan C.P.B., Wang H., Zeng Q. Monoclonal antibodies target intracellular PRL phosphatases to inhibit cancer metastases in mice. Cancer Biol. Ther. 2008;7:750–757. doi: 10.4161/cbt.7.5.5764. [DOI] [PubMed] [Google Scholar]
- 6.Guo K., Li J., Tang J.P., Tan C.P.B., Hong C.W., Al-Aidaroos A.Q.O., Varghese L., Huang C., Zeng Q. Targeting intracellular oncoproteins with antibody therapy or vaccination. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002296. [DOI] [PubMed] [Google Scholar]
- 7.Thura M., Al-Aidaroos A.Q.O., Yong W.P., Kono K., Gupta A., Lin Y.B., Mimura K., Thiery J.P., Goh B.C., Tan P., et al. PRL3-zumab, a first-in-class humanized antibody for cancer therapy. JCI Insight. 2016;1 doi: 10.1172/jci.insight.87607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thura M., Al-Aidaroos A.Q., Gupta A., Chee C.E., Lee S.C., Hui K.M., Li J., Guan Y.K., Yong W.P., So J., et al. PRL3-zumab as an immunotherapy to inhibit tumors expressing PRL3 oncoprotein. Nat. Commun. 2019;10:2484. doi: 10.1038/s41467-019-10127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thura M., Ye Z., Al-Aidaroos A.Q., Xiong Q., Ong J.Y., Gupta A., Li J., Guo K., Ang K.H., Zeng Q. PRL3 induces polypoid giant cancer cells eliminated by PRL3-zumab to reduce tumor relapse. Commun. Biol. 2021;4:923. doi: 10.1038/s42003-021-02449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funato Y., Yoshida A., Hirata Y., Hashizume O., Yamazaki D., Miki H. The Oncogenic PRL Protein Causes Acid Addiction of Cells by Stimulating Lysosomal Exocytosis. Dev. Cell. 2020;55:387–397.e8. doi: 10.1016/j.devcel.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Hong C.W., Zeng Q. Tapping the treasure of intracellular oncotargets with immunotherapy. FEBS Lett. 2014;588:350–355. doi: 10.1016/j.febslet.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Guo K., Tang J.P., Jie L., Al-Aidaroos A.Q.O., Hong C.W., Tan C.P.B., Park J.E., Varghese L., Feng Z., Zhou J., et al. Engineering the first chimeric antibody in targeting intracellular PRL-3 oncoprotein for cancer therapy in mice. Oncotarget. 2012;3:158–171. doi: 10.18632/oncotarget.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrone S. Hidden immunotherapy targets challenge dogma. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner L.M., Murray J.C., Shuptrine C.W. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–1084. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanford S.M., Bottini N. Targeting protein phosphatases in cancer immunotherapy and autoimmune disorders. Nat. Rev. Drug Discov. 2023;22:273–294. doi: 10.1038/s41573-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Aidaroos A.Q.O., Zeng Q. PRL-3 phosphatase and cancer metastasis. J. Cell. Biochem. 2010;111:1087–1098. doi: 10.1002/jcb.22913. [DOI] [PubMed] [Google Scholar]
- 17.Chee C.E., Ooi M., Lee S.C., Sundar R., Heong V., Yong W.P., Ng C.H., Wong A., Lim J.S.J., Tan D.S.P., et al. A Phase I, First-in-Human Study of PRL3-zumab in Advanced, Refractory Solid Tumors and Hematological Malignancies. Target. Oncol. 2023;18:391–402. doi: 10.1007/s11523-023-00962-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Providence, S.J.H.M.G. (2022). Bringing Breakthroughs in Cancer from the Lab to the Bedside. https://www.providence.org/news/uf/679790952?streamId=4773679.
- 19.KEYNOTE-059 (2022). A Study of Pembrolizumab (MK-3475) in Participants With Recurrent or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma https://clinicaltrials.gov/ct2/show/results/NCT02335411?view=results.
- 20.KEYNOTE-158 (2023). Study of Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-3475-158/KEYNOTE-158). https://clinicaltrials.gov/study/NCT02628067.
- 21.NCT03438396 (2022). A Trial of Tisotumab Vedotin in Cervical Cancer. https://clinicaltrials.gov/study/NCT03438396?cond=NCT03438396&rank=1.
- 22.KEYNOTE-010 (2021). Superior Overall Survival With KEYTRUDA vs Docetaxel in Second-line+ mNSCLC With PD-L1 Expression (TPS ≥1%). https://clinicaltrials.gov/study/NCT01905657.
- 23.NCT01607957 (2020). Study of TAS-102 in Patients With Metastatic Colorectal Cancer Refractory to Standard Chemotherapies (RECOURSE). https://clinicaltrials.gov/study/NCT01607957.
- 24.Janjigian Y.Y., Shitara K., Moehler M., Garrido M., Salman P., Shen L., Wyrwicz L., Yamaguchi K., Skoczylas T., Campos Bragagnoli A., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 26.A'Hern R.P. Sample size tables for exact single-stage phase II designs. Stat. Med. 2001;20:859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 27.Suissa S. Single-arm Trials with Historical Controls: Study Designs to Avoid Time-related Biases. Epidemiology. 2021;32:94–100. doi: 10.1097/EDE.0000000000001267. [DOI] [PubMed] [Google Scholar]
- 28.Krebs M.G., Blay J.Y., Le Tourneau C., Hong D., Veronese L., Antoniou M., Bennett I. Intrapatient comparisons of efficacy in a single-arm trial of entrectinib in tumour-agnostic indications. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Huo M., Zhou J., Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010;99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. This paper does not report any original code. Any additional information required to reanalyze the data reported in this paper is available from lead contact upon request.