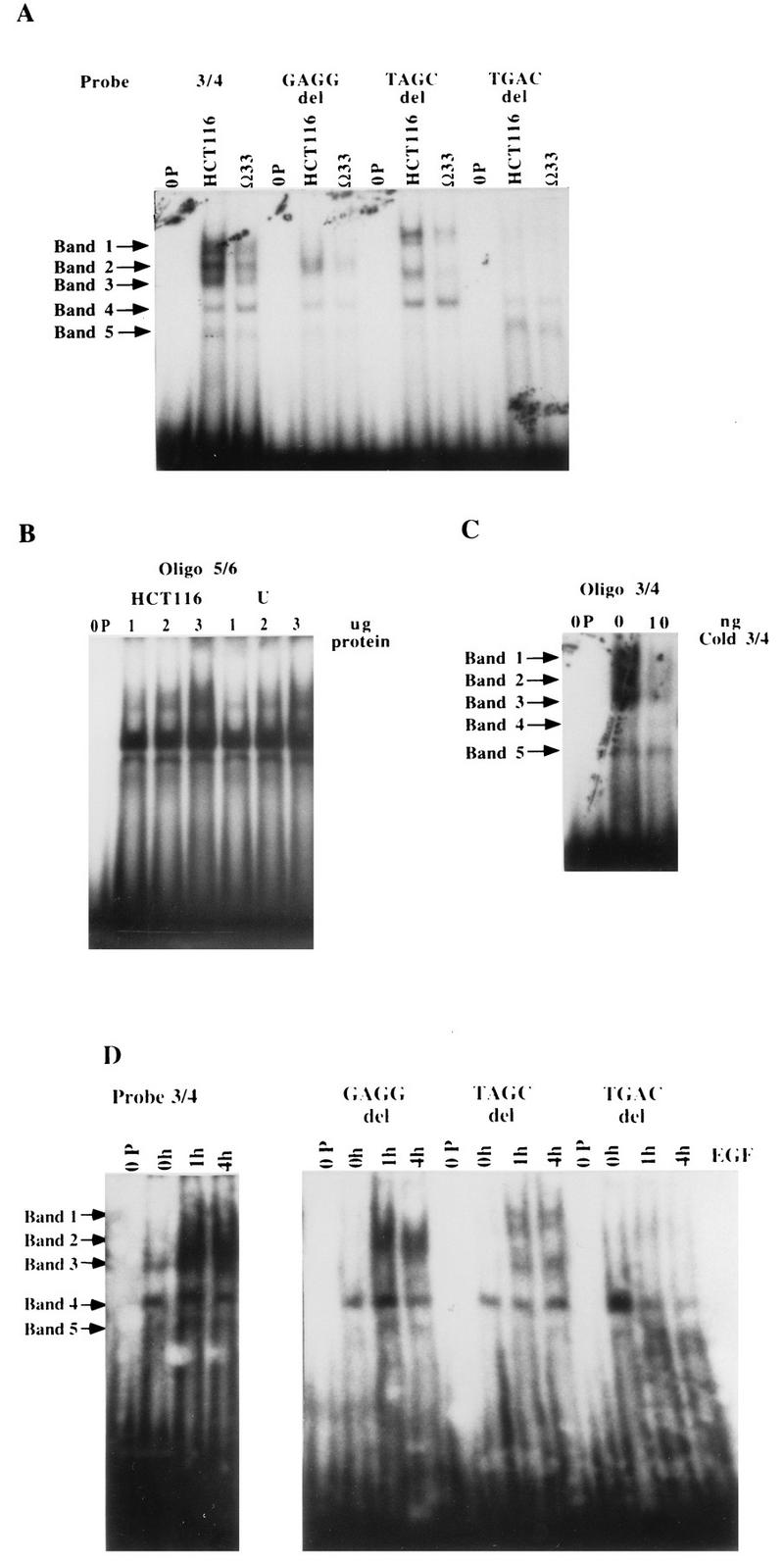
FIG. 8.
Gel shift assay with the TGFα autoregulatory element. (A) Gel shift analysis of complexes formed with the TGFα autoregulatory element (oligonucleotide 3/4 [3/4]) and the GAGG, TAGC, and TGAC deletions (del) of this element. Equivalent amounts of nuclear extract protein (3 μg) from HCT116 control cells and TGFα antisense clone 33 were run against the various 32P-labelled oligonucleotides. 0P denotes the lanes containing probe run without protein; Ω33 denotes the lanes containing probe run with clone 33. (B) Gel shift analysis of complexes formed with control oligonucleotide 5/6. Equivalent amounts of nuclear extract protein (3 μg) from control HCT116 cells and TGFα antisense cells were run against 32P-labelled oligonucleotide 5/6, which contains −201 to −176 of the TGFα promoter sequence. This sequence does not participate in EGF or TGFα regulation and does not confer EGF or TGFα responsiveness to a heterologous-promoter construct. (C) Specificity of nuclear protein binding to the TGFα autoregulatory element. Nuclear extract (3 μg protein) was run against 32P-labelled oligonucleotide 3/4 in the presence (10 ng) or absence (0 ng) of cold competing oligonucleotide 3/4 (Cold 3/4). (D) Effect of exogenous EGF on nuclear protein binding in TGFα antisense clone 33. Equivalent amounts of nuclear extract protein from TGFα antisense clone 33 cells maintained without EGF or treated with EGF (10 ng/ml) for 1 or 4 h prior to harvest were run against the 32P-labelled TGFα autoregulatory element (oligonucleotide 3/4) or the GAGG, TAGC, and TGAC deletion oligonucleotides described in the legend to Fig. 7. 0h, 1h, and 4h denote the durations of EGF treatment.