FIG. 8.
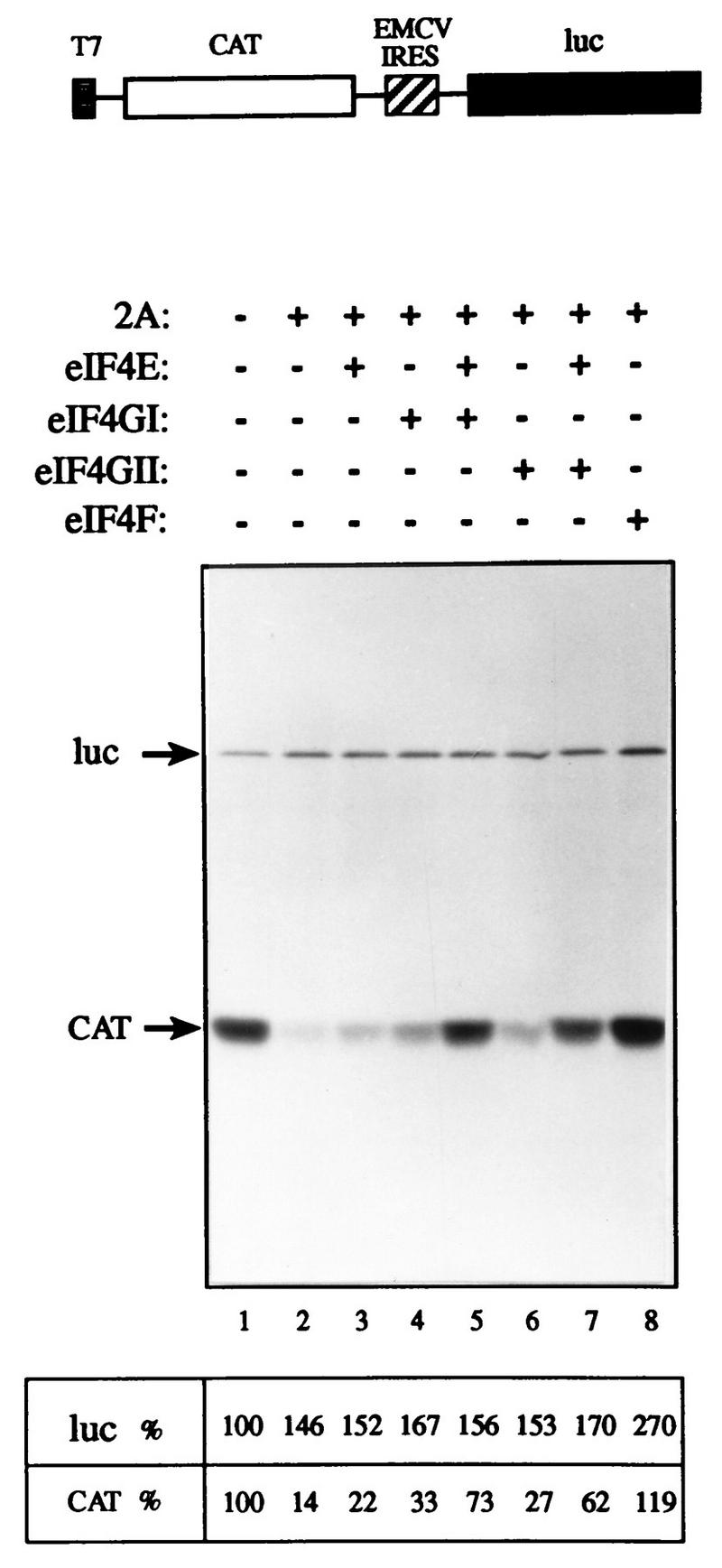
eIF4GII restores cap-dependent translation to an eIF4G-deficient extract. Rabbit reticulocyte lysate (90 μl) was mock treated (lane 1) or treated with 3.6 μg of rhinovirus 2Apro (13) (lanes 2 to 8) for 5 min at 30°C, followed by a 10-min incubation on ice in the presence of 0.8 mM elastatinal (Sigma). Aliquots (12.5 μl) were programmed for translation with 0.1 μg of capped bicistronic pGEMCAT/EMC/LUC mRNA (shown schematically at the top of the figure) in the presence of [35S]methionine and supplemented with the indicated initiation factors as follows: lanes 1 and 2, buffer alone; lane 3, 0.05 μg of eIF4E; lane 4, 0.45 μg of eIF4GI; lane 5, 0.05 μg of eIF4E and 0.45 μg of eIF4GI; lane 6, 0.5 μg of eIF4GII; lane 7, 0.05 μg of eIF4E and 0.5 μg of eIF4GII; lane 8, 0.5 μg of rabbit reticulocyte eIF4F. Translation and processing for electrophoresis were conducted as described in Materials and Methods. Following autoradiography, the bands corresponding to luciferase (luc) and CAT were quantified densitometrically. The efficiency of translation of the luciferase and CAT products is given as a percentage of that of the control (lane 1). The positions of the luciferase and CAT proteins are indicated to the left by arrows.
