FIG. 1.
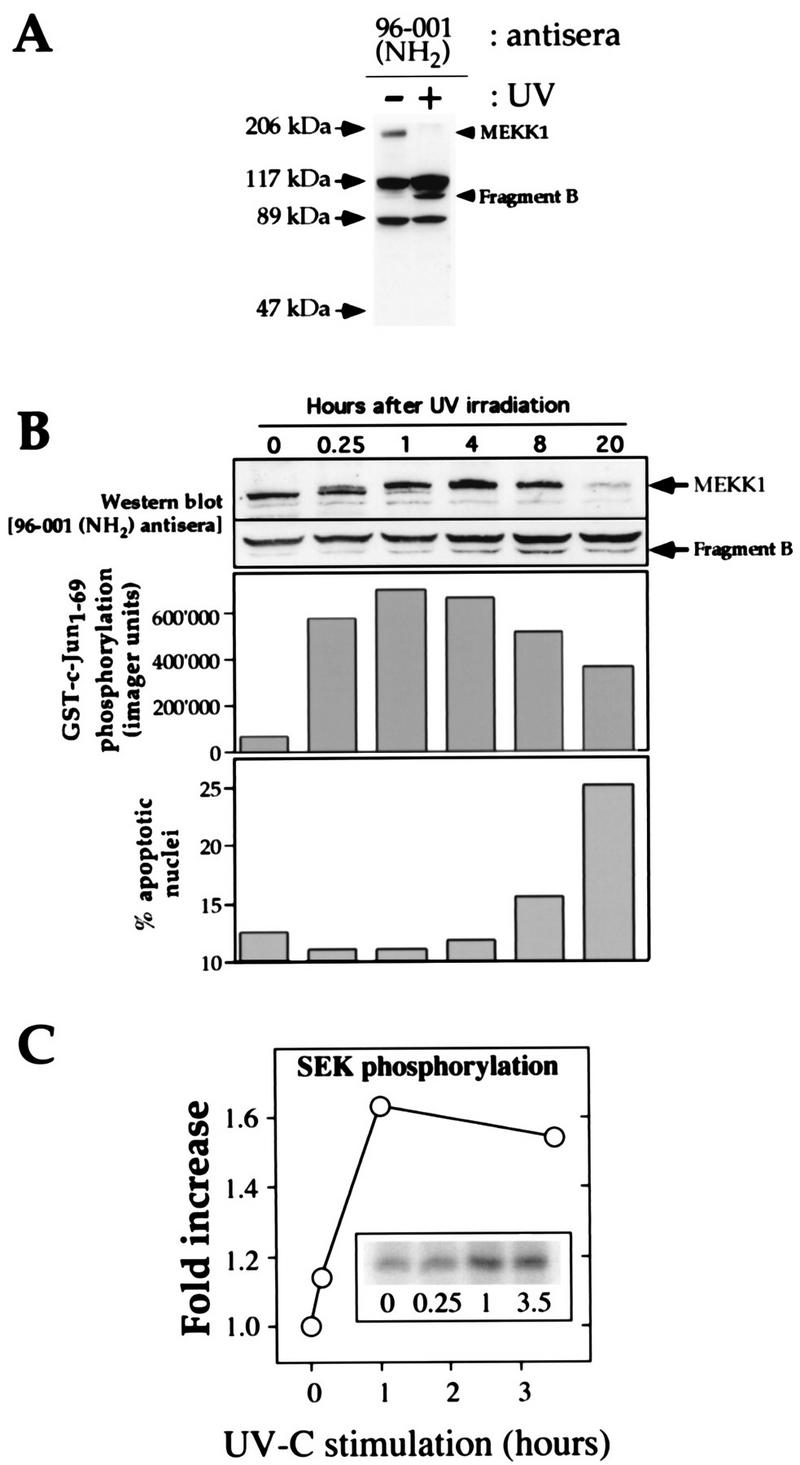
UV induces rapid phosphorylation of endogenous MEKK1 in HEK293 cells, followed by the cleavage of MEKK1 into smaller fragments. (A) HEK293 cells were irradiated or were not irradiated with UV (100 J/m2) and incubated for 16 h in DMEM containing 0.1% serum. The cells were then lysed and subjected to Western blot analysis with the MEKK1 NH2-terminus-specific antibody 96-001. The positions of full-length MEKK1 and the UV-generated NH2-terminal fragment (fragment B) are indicated. (B) The cells were treated with UV (100 J/m2) and incubated for the indicated times in DMEM containing 0.1% serum. (Top panel) Cell lysates were analyzed as described in the legend to Fig. 1A. For clarity, only the portions of the gel containing the 196-kDa MEKK1 protein and the immunoreactive MEKK1-derived fragment are shown. (Middle panel) Activation of the JNK pathway was measured with Sepharose-bound GST-Jun as a substrate. (Bottom panel) Propidium iodide-stained nuclei were analyzed with a fluorescence-activated cell sorter (46). The percentage of nuclei with an altered shape (apoptotic nuclei) was plotted as a function of time. (C) The cells were treated as in panel B. The kinase activity of the endogenous MEKK1 protein in response to UV-C irradiation was measured as described in Materials and Methods. The activation of MEKK1 in response to UV-C irradiation temporally correlated with its gel shift and with the activation of the JNK pathway.
