Abstract
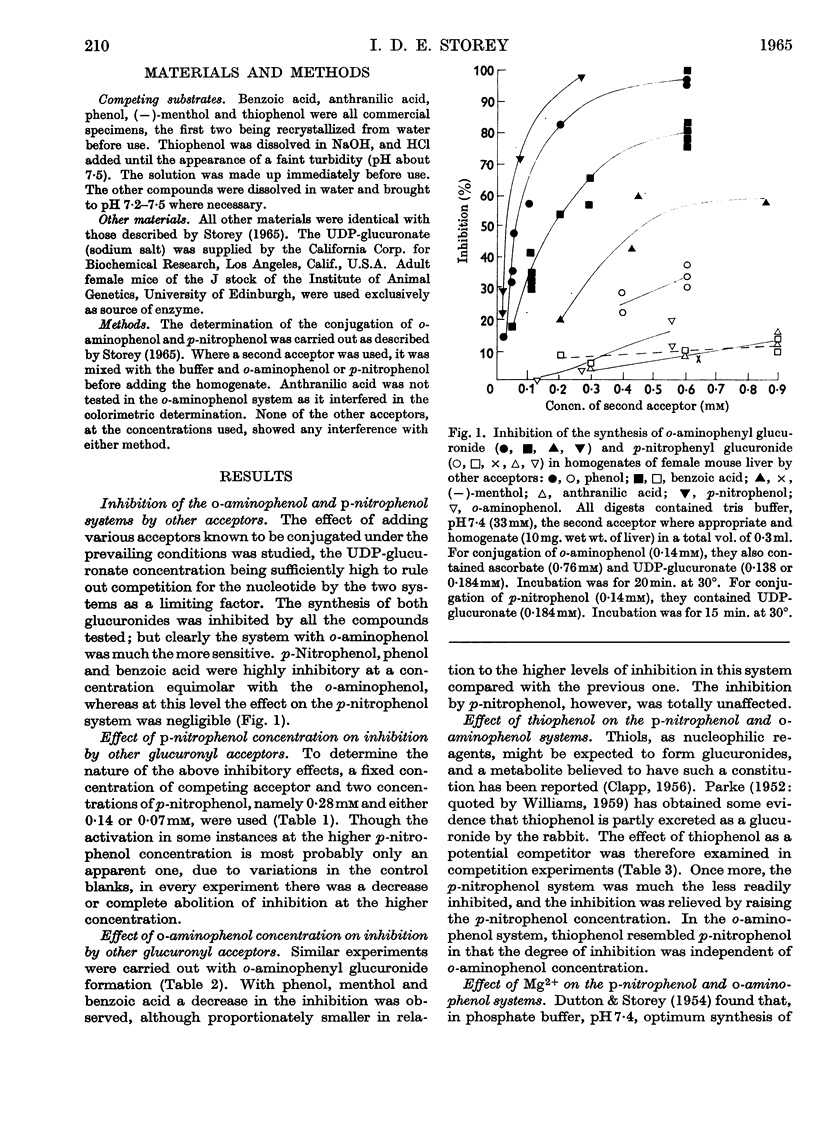
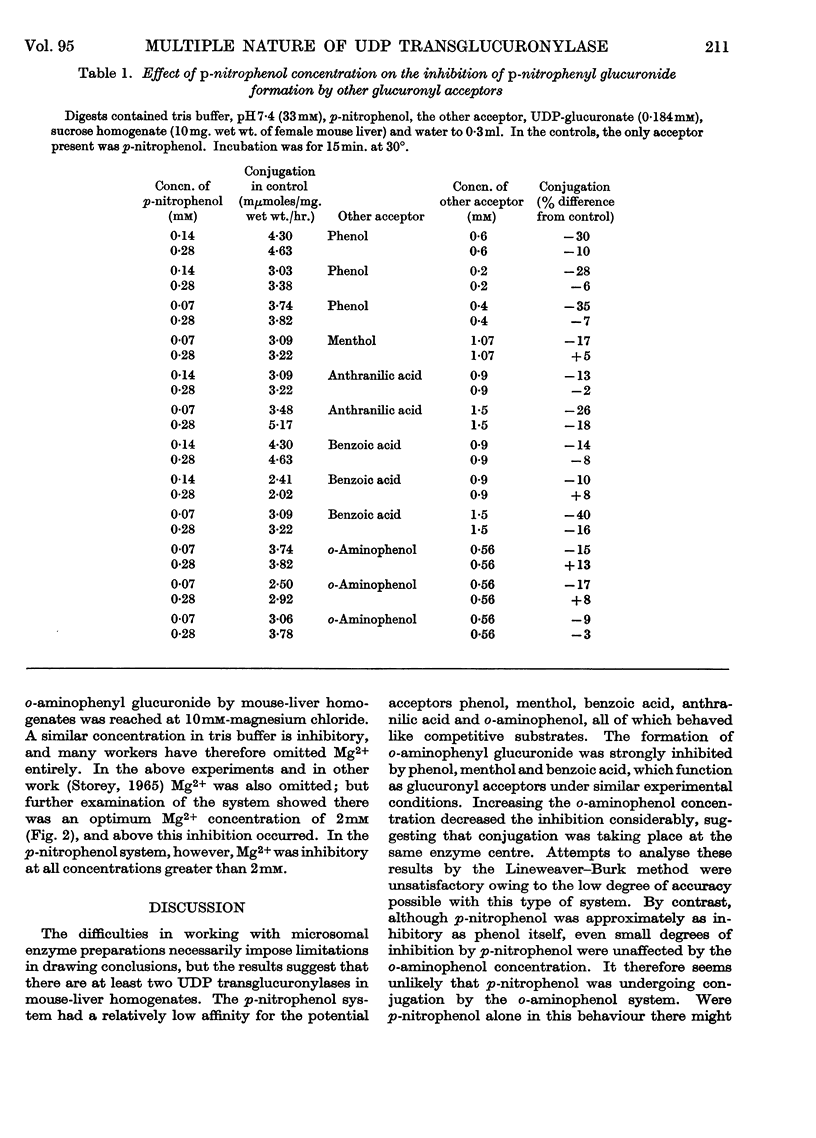
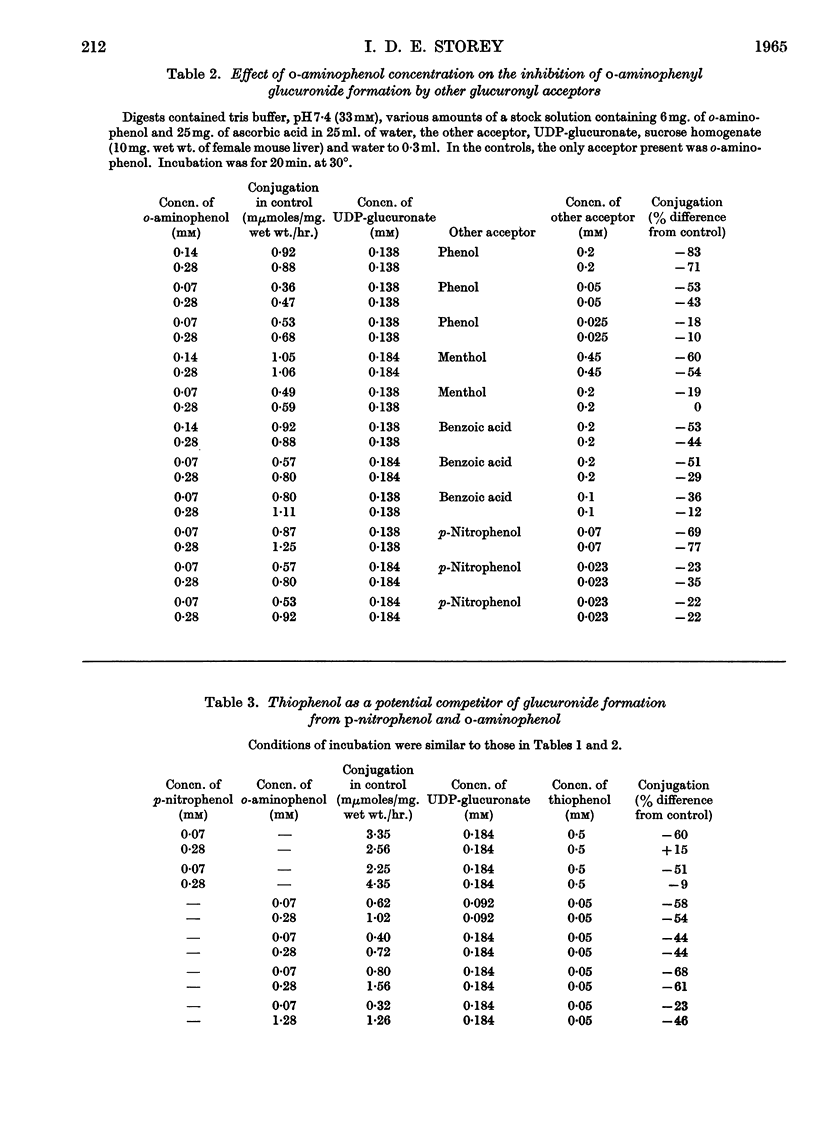
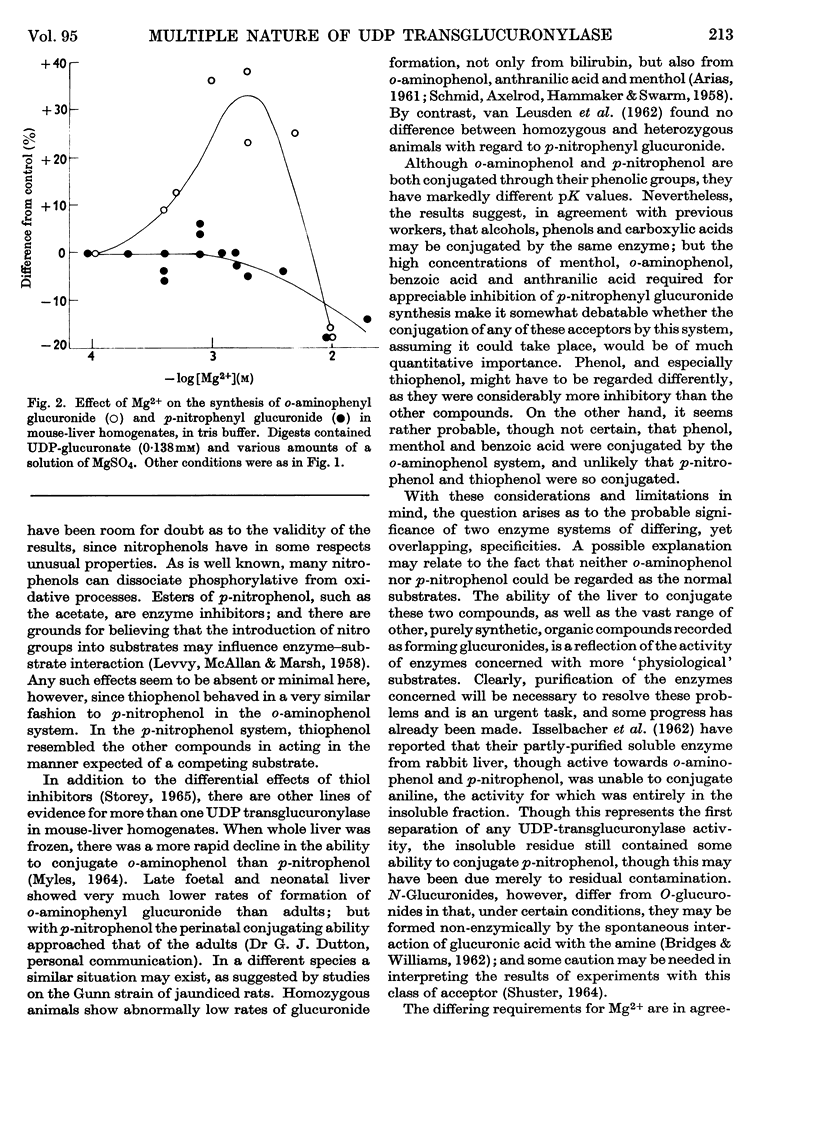
1. Glucuronide synthesis from uridine diphosphate glucuronate and o-aminophenol or p-nitrophenol in the presence of uridine diphosphate transglucuronylase of mouse-liver homogenates has been studied with respect to inhibition by compounds known to be conjugated under the experimental conditions, and also by thiophenol. 2. Raising the o-aminophenol concentration decreased the inhibition of o-aminophenyl glucuronide synthesis by the alternative glucuronyl acceptors phenol, menthol and benzoic acid, but was without effect on that caused by p-nitrophenol and thiophenol. 3. Raising the p-nitrophenol concentration decreased or abolished the inhibition of p-nitrophenyl glucuronide synthesis due to phenol, menthol, benzoic acid, anthranilic acid, o-aminophenol and thiophenol. 4. The o-aminophenol system was much more readily inhibited by all compounds than the p-nitrophenol system. 5. In tris buffer, pH7·4, over 30% activation of the o-aminophenol system was achieved by 2mm-Mg2+, but 10mm-Mg2+ was inhibitory. The p-nitrophenol system showed only inhibition from 2mm-Mg2+ upwards. 6. The results are discussed as suggesting that there are at least two uridine diphosphate transglucuronylases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., INSCOE J. K., TOMKINS G. M. Enzymatic synthesis of N-glucosyluronic acid conjugates. J Biol Chem. 1958 Jun;232(2):835–841. [PubMed] [Google Scholar]
- CLAPP J. W. A new metabolic pathway for a sulfonamide group. J Biol Chem. 1956 Nov;223(1):207–214. [PubMed] [Google Scholar]
- DUTTON G. J. COMPARISON OF GLUCURONIDE SYNTHESIS IN DEVELOPING MAMMALIAN AND AVIAN LIVER. Ann N Y Acad Sci. 1963 Dec 30;111:259–273. doi: 10.1111/j.1749-6632.1963.tb36967.x. [DOI] [PubMed] [Google Scholar]
- DUTTON G. J., STOREY I. D. Uridine compounds in glucuronic acid metabolism. I. The formation of glucuronides in liver suspensions. Biochem J. 1954 Jun;57(2):275–283. doi: 10.1042/bj0570275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTTON G. J. Uridine diphosphate glucuronic acid as glucuronyl donor in the synthesis of ester, aliphatic and steroid glucuronides. Biochem J. 1956 Dec;64(4):693–701. doi: 10.1042/bj0640693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., CARBONE J. V. The synthesis of bilirubin glucuronide by tissue homogenates. J Biol Chem. 1957 May;226(1):449–458. [PubMed] [Google Scholar]
- ISSELBACHER K. J., CHRABAS M. F., QUINN R. C. The solubilization and partial purification of a glucuronyl transferase from rabbit liver microsomes. J Biol Chem. 1962 Oct;237:3033–3036. [PubMed] [Google Scholar]
- LEVVY G. A., McALLAN A., MARSH C. A. Purification of beta-glucuronidase from the preputial gland of the female rat. Biochem J. 1958 May;69(1):22–27. doi: 10.1042/bj0690022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID R., AXELROD J., HAMMAKER L., SWARM R. L. Congenital jaundice in rats, due to a defect in glucuronide formation. J Clin Invest. 1958 Aug;37(8):1123–1130. doi: 10.1172/JCI103702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUSTER L. METABOLISM OF DRUGS AND TOXIC SUBSTANCES. Annu Rev Biochem. 1964;33:571–596. doi: 10.1146/annurev.bi.33.070164.003035. [DOI] [PubMed] [Google Scholar]
- STOREY I. D. THE INHIBITION OF THE URIDINE DIPHOSPHATE-TRANSGLUCURONYLASE ACTIVITY OF MOUSE-LIVER HOMOGENATES BY THIOL REAGENTS. Biochem J. 1965 Apr;95:201–208. doi: 10.1042/bj0950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van LEUSDEN H., BAKKEREN J. A., ZILLIKENF, STOLTELA p-Nitrophenylglucuronide formation by homozygous adult Gunn rats. Biochem Biophys Res Commun. 1962 Feb 20;7:67–69. doi: 10.1016/0006-291x(62)90147-x. [DOI] [PubMed] [Google Scholar]


