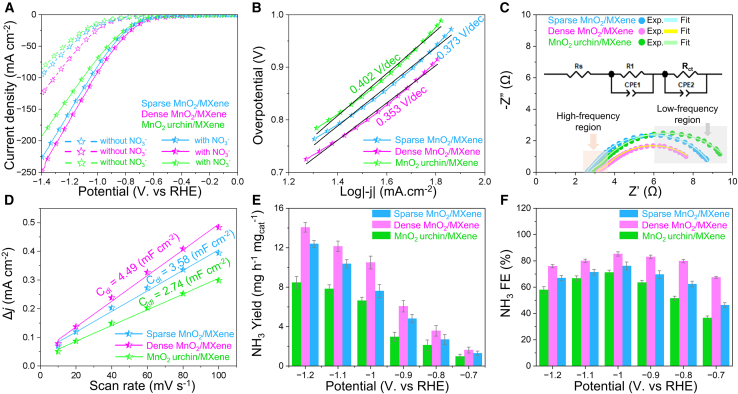
Figure 4.
Electrochemical evaluation of as-prepared catalysts for NO3RR
(A) Linear sweep voltammetry (LSV) at 5.0 mV s−1 in 0.5 M K2SO4 with and without 0.1 M KNO3, In 0.5 M K2SO4 and 0.1 M KNO3 electrolyte: (B) corresponding Tafel plot of nitrate reduction kinetics; (C) Electrochemical impedance spectroscopy (EIS) with 5 mV alternating current amplitude at an applied potential of −0.6 V vs. RHE; (D) Double-layer capacitance (Cdl) measurement for determining electrochemically active surface area; (E) Faradaic efficiency (FE) and (F) yield rate of NH3 as a function of overpotential for different catalysts. Error bars represent the standard deviation in yield rate and FE calculated after three tests for repeatability.