Research highlights
-
•
ME-CDBD used to prepare plasma-treated water (PTW) and PTW with metal ion (PTW+M).
-
•
PTW and PTW+M increased the salt tolerance in Pak Choi.
-
•
RNS and Ca2+ levels increased after PTW and PTW+M treatments in Pak Choi.
-
•
Salt overly sensitive (SOS) pathway was regulated to improve Pak Choi growth.
-
•
PTW and PTW+M treatments improved the accumulation of primary metabolites.
Keywords: Multi-electrode cylindrical dielectric barrier discharged plasma, PTW with metal ions, NaCl solution, Photosynthetic activity, Intracellular NO and Ca2+ level, Salt tolerance genes
Abstract
Salinity stress is a serious environmental threat that has a detrimental impact on agricultural yield and productivity. The effects of plasma-treated water (PTW) and metal ions (M) on Pak Choi growth and salt tolerance genes under salinity stress have not yet been studied. To bridge these gaps, we explored the effects of PTW and PTW + M, prepared using multi-electrode cylindrical dielectric barrier discharged plasma, on Pak Choi seedlings irrigated with 100 mM NaCl solution for 7 d, with an aim to improve plant tolerance to salinity. PTW and PTW + M significantly improved the length and dry weight of shoots and roots, as well as photosynthetic activity, by increasing chlorophyll a and b, pheophytin a and b, total pheophytin, and carotenoid contents. Plant biomass increased maximum of up to 54.4%, shoot length 18.1%, and root length 26.01% in the PTW + M group compared to the control. Furthermore, total chlorophyll and pheophytin content increased 2.16 and 1.55 times after PTW + M irrigation compared to control (DI irrigation) under salinity stress. PTW and PTW + M irrigation further reduced the plasma membrane permeability along with an increase in hydration and intracellular NO and Ca2+ levels, with ionic balance and osmotic adjustment genes under salinity stress. Additionally, the treatments improved the primary metabolite accumulation of sugars, sugar alcohols, organic acids, and amino acids, as osmo-protectants, and modulated the adaptation to mitigate salinity stress. In conclusion, PTW and PTW + M irrigation mitigated salinity stress by promoting osmotic adjustment, membrane impermeability, hydration capacity, and the upregulation of salt tolerance genes and primary metabolites in Pak Choi seedlings under salinity stress.
Graphical abstract
1. Introduction
Environmental factors have many detrimental effects including drought, salinity, and the rise of worldwide temperatures which has noticeably reduced agricultural production and the worldwide availability of food [1,2]. In general, salinization damages more than 800 million hectares of land; additionally, 20% of farmland is damaged by salinity stress, which is increasing each year because of excessive spraying of fertilizers and pesticides. This further decrease or hinders plant production. By 2050, salinization damage is expected to increase by 50% [[3], [4], [5]]. Among the various environmental stresses, salinity is the most damaging stress that restricts plant growth and development because of excessive accumulation of sodium ions (Na+), which are not required for promoting plant growth. Na+ also hinders the entry of potassium ions (K+), which is crucial for enzymatic activity during plant growth [[6], [7], [8], [9]]. The influx of neutral ions, particularly NaCl and Na2SO4, causes salinity stress. Plants are negatively influenced by sodium and chloride ions; particularly, sodium ions in the soil decrease water availability [10].
Excessive salt concentrations cause oxidative damage, osmotic stress, and ion toxicity, and inhibit other biochemical and molecular processes. These negative salinity stresses generally affect germination, seedling stage, vegetative growth, and flowering [11]. Genes in the salt overly sensitive (SOS) pathway play a role in Na+ extrusion; particularly, SOS1, SOS2, and SOS3 promote ion homeostasis for cellular signaling under salinity stress in plants [12]. The SOS pathway is induced by salt stress and mediates the vacuolar Na+/H+ exchange in Arabidopsis [13]. Furthermore, AtNHX proteins are responsible for the regulatory pathway components SOS1 in tonoplast (vacuolar membrane) Na+/H+ exchange [14]. Nitric oxide (NO) is a well-known reactive nitrogen species with specific signaling functions in plant growth and development [15,16]. Additionally, the exogenous application of NO modulates the stomatal closure of plants to prevent transpiration under drought stress [17]. NO as a signaling molecule stimulates the activation of various antioxidant systems and biosynthetic signaling pathways to improve salt tolerance mechanisms, such as the SOS pathway [6]. Many techniques, such as genetic breeding, seed priming, application of bio stimulants and osmoprotectants, and addition of exogenous stimulants with adjustments in efficacy degrees, have been used to control environmental stresses [[18], [19], [20], [21], [22]].
Recently, nonthermal atmospheric pressure plasma (NAPP) has become a cutting-edge, environmentally friendly technology for universal seed culture methods [23,24]. The effectiveness of different NAPP techniques on plasma from seeds to fields has been discussed in the available literature on agriculture [[25], [26], [27]]. The availability of water treated with discharged plasma significantly affects seed germination and seedling development. Owing to its widespread application in the agricultural industry, direct NAPP treatment is not sufficient for agricultural production. Hence, an indirect method for the production of plasma-treated water (PTW) has been introduced [28]. Irrigating plants with PTW increases agricultural yield [29,30]. Moreover, PTW can produce various reactive oxygen and nitrogen species (RONS) that are essential for improving plant physiology and growth. Long-lived reactive oxygen and nitrogen species (H2O2, NO2, and NO3) in PTW are reliable and eco-friendly substitutes for fertilizers to increase agricultural yields [31,32]. To overcome unfavorable conditions, such as salinity, PTW could be a promising technology for inducing plant tolerance [31,33]. PTW can be applied during the germination stages to promote stress tolerance to low temperatures and hypoxic stressors by controlling the levels of carotenoids, cysteine, and glutamyl cysteine under salinity [[34], [35], [36]].
Pak Choi (Brassica rapa subsp. Chinensis L.) is an Asian leafy vegetable belonging to the Brassicaceae family [37]. In our previous experiment, we used plasma-treated water (PTW) with low pH (∼3–4) and neutralized the pH of PTW by immersing metals symbolized (M: Mg2+ and Zn2+) during PTW formation. So, the plasma-treated water was symbolized “PTW” and PTW with metal ions (Mg2+ and Zn2+) was indicated by “PTW + M”. We studied the effect of PTW with metal ions (Mg2+ and Zn2+) on the germination and growth of Pak Choi [38]. However, the cultivar of Pak Choi seedlings faces the risk of salinity stress and the effects of PTW and PTW + M on Pak Choi growth under salinity stress have not yet been studied. To address these gaps, we examined the impact of PTW and PTW + M, prepared using multi-electrode cylindrical dielectric barrier discharge plasma, on Pak Choi seedlings irrigated with 100 mM NaCl solution for 7 days, aiming to enhance salinity tolerance. For the recovery treatments, plants were irrigated with deionized (DI) water (control: without salt stress), DI water (under salinity stress), PTW (under salinity stress), and PTW + M (under salinity stress) for harvesting periods of 3, 6, and 9 d. The reactive species in PTW and PTW + M played a significant role in enhancing the tolerance of Pak Choi under salinity stress. Furthermore, we first examined the effect of PTW and PTW + M on Pak Choi seedlings under salinity stress at physiological, biochemical, molecular, and metabolite levels.
2. Materials and methods
2.1. ME-CDBD plasma source and Fourier transform infrared (FTIR) analysis
In this study, an ME-CDBD source was used to produce plasma. The plasma source consisted of six brass electrodes, each of which was inserted into a quartz tube. The diameter of each brass electrode (20 mm) was the same as the inner diameter of the quartz tube. The brass electrodes inside the quartz tubes were 170 mm long. All electrodes and quartz tubes were inserted into the outer quartz tubes (inner diameter: 90 mm; length: 25 cm). Of the six brass electrodes, three were connected to a high voltage alternating current source, and three were connected to the ground. Air was used as feed gas. The plasma-generated reactive nitrogen species (RNS) was quantified by a Burker MATRIX-MG gas transmission FTIR spectrometer, which was equipped with a liquid nitrogen-cooled MCT detector, covering a spectral range of 600–4,800 cm−1 and with a resolution of 1 cm−1.
2.2. Raman spectroscopy and physiochemical properties of irrigated water
When an alternating current source (3 kV, 20 kHz) was applied to the high-voltage electrode in air, the plasma-generated reactive species inside the tubes were delivered and dissolved in the water. The generated plasma was directly mixed with 1,000 mL of DI water to produce PTW. For pH neutralization, 4 g of metals (Mg2+ and Zn2+) represented as “M” were used during plasma treatment by continuous stirring at 450 rpm to form Mg2+ and Zn2+ ions from the respective metal oxides. The plasma on time was 60 min in both treated conditions. The bonds formed between the water molecules and dry zinc and magnesium metals were identified by WITec alpha 300 Raman spectroscopy using a laser with an excitation wavelength of 488 nm and with 50 × magnification. The laser power was fixed at 3 milliwatts and the integration time was maintained at 5 s, with 15 accumulations.
We also measured the physicochemical properties of the various types of water used in our experiments, including PTW and PTW + M. The concentration of H2O2 and NOx were measured using QuantiChromTM peroxidase and NO assay kits, respectively. Nitrite (NO2−) and nitrate (NO3−) levels were measured using the Bio Vision nitric oxide assay kit according to the manufacturer's instructions. The pH was measured with the aid of a pH spear (Eutech Instruments, Paisley, United Kingdom), while electrical conductivity (EC) and total dissolved solids (TDS) were measured by the portable OAKTON PCTS TESTR™ 50, and oxidation reduction potential (ORP) was measured by the ORP30 Tester (Clean Instruments) in all Pak Choi irrigation experiments.
2.3. Growth conditions
Pak Choi seeds (Brassica rapa subsp. Chinensis L.) were purchased from Seed Industry Co., Ltd. (Korea). The seeds were selected based on their uniformity in size, shape, and color. All seeds were surface sterilized for 10 min in a 1% (v/v) sodium hypochlorite (NaClO) solution and thoroughly washed with DI water. They were then soaked in DI water in plant culture dishes covered with cotton. Each dish contained 50 seeds in triplicates. The fully unfolded Pak Choi cotyledons were defined as the emergence radical (embryonic root) and plumule (embryonic shoot). After 7 d, 3–4 seedlings were implanted into filled vermiculite pots (50 g capacity) in an illuminating growth incubator (16 h light with a light intensity of 245 µmol m−2 s−1, Philips Fluorescence Tube Light Bulb, 25 ± 1 °C, and 8 h dark, 23 ± 1° C, 60% relative humidity) for two weeks and irrigated daily with 10 mL DI. Moreover, half-strength Hoagland solution was added alternatively twice a week.
2.4. Salinity stress and recovery treatments of Pak Choi
Initially, the Pak Choi seedlings were subsequently treated with four different NaCl levels (50, 100, 150, and 200 mM) for 7 days (data not provided). We observed the physiological indicators, and visual symptoms of stress under controlled environmental conditions by adjusting irrigation with a gradual increase of NaCl concentration. After day 7, we observed from our preliminary experiment that a 100 mM concentration of NaCl is optimum for our study design. The control and saline stress treatment are watered once a day for 7 continuous days with fixed water volume and NaCl concentrations to ensure the stability of salt stress. Once the symptoms of salinity stress are visible, we have subjected them to DI, PTW, and PTW + M irrigation for recovery up to 9 days. Three-week-old uniform seedlings were selected from two groups: (i) normal control wherein seedlings were grown with only DI water (10 mL/pots), and (ii) salinity stress treatment wherein seedlings were irrigated with 100 mM NaCl (10 mL/pots) for 7 d. The normal control (DI) and salinity-treated plants irrigated with 100 mM NaCl were watered daily. Subsequently, along with the normal control (DI), the salinity- stressed seedlings were divided into three groups based on different recovery treatments: (1) Salinity control (seedlings irrigated with 10 mL DI water alone, (2) salinity pretreated (100 mM NaCl) (seedlings irrigated with 10 mL freshly prepared PTW), and (3) salinity pretreated (100 mM NaCl) (seedlings irrigated with 10 mL of freshly prepared PTW + M). Shoot and root samples were collected on days 3, 6 and 9 after treatment for physiological, biochemical, molecular, and primary metabolite analysis. Each analysis consisted of 60 pots for two independent experiments. Three to four Pak Choi seedlings were grown in each pot under four conditions, including the normal control (DI) (Fig. 1).
Fig. 1.
Graphical representation of treatment methodology. First, the plant was grown for 14 d. Second, the plant was subjected to salt stress for 7 d. Lastly, recovery treatment was provided for 3, 6, and 9 d as follows: DI (control: without salinity stress), DI, PTW, and PTW + M (with salinity stress).
2.5. Growth parameters after recovery treatments
Shoot and root lengths of Pak Choi plants were measured in DI, NaCl, PTW, and PTW + M treatments on a centimeter scale, at the end of days 3, 6, and 9. The fresh weights of shoots and roots under each condition were calculated on each harvest day. Moreover, the separated shoot and root samples were dehydrated at 65 °C for 5 d in a dry oven until the sample weight stabilized. The dry weight of each sample was also measured with respect to the harvesting time.
2.6. Chlorophyll, pheophytin, and total carotenoid content measurements
Chlorophyll is present in the chloroplasts and plays an essential role in light harvesting for photosynthesis. Fresh leaves were weighed equally (200 mg), homogenized with acetone (80%), and centrifuged at 3,000 rpm for 10 min. Until all the chlorophyll was extracted into the solvent, the supernatant was transferred to new Falcon tubes and centrifuged again. Using acetone (80%), the mixed supernatant was brought to a defined volume. Moreover, using acetone as a blank, the absorbance OD values of the extract were measured at 663 and 646 nm for chlorophyll a and b, respectively, denoted by and respectively. For carotenoids, a wavelength of 470 nm (A470) was used for absorption and wavelengths of 665, 653, and 470 nm were used for pheophytins a, b, and total pheophytins, respectively [39,40]. For chlorophyll detection, (1), (2), (3), (4) were used.
| (1) |
| (2) |
| (3) |
| (4) |
where, and are the biological mass of chlorophyll a, b, and their total amount of chlorophyll, respectively. Additionally, was denoted as carotenoid in terms of chlorophyll. For pheophytin detection, (5), (6), (7), (8) were used.
| (5) |
| (6) |
| (7) |
| (8) |
where , and are biological mass of pheophytin a, b and their total amount of pheophytin, respectively. Also, is the amount of carotenoid in terms of pheophytin.
2.7. Determination of relative water content (RWC) and electrolyte leakage (EL)
RWC was determined following Ahmed et al. [41] and according to Eq. 9. The fresh weight of Pak Choi leaves was immediately measured after harvesting on days 3, 6, and 9. Subsequently, all leaves were soaked in equal volumes of water at 25 °C room temperature for 15 h in the dark, and turgid weight was measured. Leaf samples were then oven-dried at 65 °C for 48 h for dry weight measurements.
| (9) |
EL was measured following Wu et al. [42] with slight modifications and according to Eq. 10. Briefly, 50 mg of fresh leaves were cut into small pieces, dipped into 10 mL of DI water, and shaken for 24 h at 25 °C room temperature to measure the electrical conductance (EC1) using the portable OAKTON PCTS TESTR™ 50. Subsequently, the samples were heated at 120 °C for 30 min, and electrical conductance (EC2) was measured using the same device.
| (10) |
2.8. Measurement of malondialdehyde (MDA) content and nitric oxide levels
MDA is a direct measure of lipid peroxidation in plants. To measure MDA, 200 mg of the leaf sample was ground in liquid nitrogen and mixed with 2 mL of 20% trichloroacetic acid containing 0.5% thiobarbituric acid. The homogenized samples were incubated at 95 °C in a water bath for 25 min. The samples were then transferred to an ice bath for cooling and centrifuged at 10,000 rpm for 10 min. Later, 150 µL of the sample and standard were loaded in a 96-well plate and fluorescence was measured at 540 nm excitation and 600 nm emission in triplets.
For NO measurement, 100 mg of fresh leaves were ground in liquid nitrogen; subsequently, 1 mL of 0.25 M sodium phosphate (pH 7.4) buffer was added. The supernatant was collected and centrifuged at 4 °C for 20 min and 10,000 rpm. The sample was mixed with the Griess reagent [1% sulfanilic acid (C6H7NO3S), 1% N-1naphylenediamin dihydrochloride (C10H8N2.2HCl) and 5% phosphoric acid (H3PO4]), and the reaction mixture was incubated for 30 min at 25 °C room temperature. To determine NO levels, absorbance was measured at 540 nm using a plate reader (Synergy BioTek, USA).
2.9. Molecular real-time quantitative polymerase chain reaction (RT-qPCR) analysis
Quantitative polymerase chain reaction (qPCR) was conducted to analyze the salt tolerance genes, following Schnittger et al. [43]. Briefly, RNA from Pak Choi leaves was isolated using RNA Iso Plus (Invitrogen, USA). To prepare cDNA, M-MLV First Strand Kit (Invitrogen) was used to prepare cDNA. Primers for salt tolerance genes, such as the salt overly sensitive (SOS) complex (SOS1, SOS2, and SOS3), CaX1, NHX1, HKT1, P5CS1, KCS1, GORK, SAL1, and AKT1 were investigated. Primers used for all genes used in this study are listed in Table S1.
2.10. Detection of intracellular reactive species and calcium levels
Leaves from salinity-stressed plants were harvested to detect intracellular RONS and calcium ions (Ca2+). The abaxial epidermis of leaves were peeled and immersed in 10 µM H2DCFDA and DAF-FM florescence dye (made in 10 mM MES-KCl buffer for measuring RONS. To detect the intracellular Ca2+, abaxial epidermis of leaves were dipped in 10 µM Fluo3-AM (Invitrogen) for 2 h at 25 °C room temperature in the dark environment. All stained and labelled samples were analyzed, and images were captured using the FV-100 MPE spectra from a confocal laser scanning microscope (Olympus Corporation) to detect RONS and Ca2+.
2.11. Extraction of primary metabolites
Pak Choi seedlings were grown under recovery treatments from salinity stress after harvesting on day 9 and were instantly placed in liquid nitrogen. Briefly, 30 mg of powdered samples were added to a 2 mL centrifuge tube, and each tube was filled with 1 mL of 70% methanol that had been pre-cooled to 20 °C. The mixtures were vortexed and ground using a 70 Hz grinding mill system. After ultrasonication extraction, they were homogenized using a 0.2 µL syringe filter. The dried samples were dissolved and derivatized for gas chromatography-mass spectrometry (GC–MS) analysis. Blank samples were prepared by using a mixture of aliquots from each biological sample in the respective analytical runs. The control samples were studied along with the actual samples.
2.12. GC–MS analysis
For the GC–MS analysis, a GC (TRACETM 1310)-MS (ISQTM LT Single Quadrupole GC/MS) equipped with a DB-5MS (Agilent J & W Scientific, Folsom, CA, USA) was used. The obtained mass spectra were exported to extensible computational mass spectrometry software for peak recognition, data baseline filtering, raw signal extraction, and integration. Finally, data in the “CSV” file was exported with sample information including retention duration and peak intensities for analysis [44].
2.13. Statistical analysis
All experiments were performed in triplicate. The student's t-test was used to determine statistically significant p values, denoted by ap < 0.05, bp < 0.01, cp < 0.001.
3. Results
3.1. Characteristics of ME-CDBD plasma and physiochemical properties of irrigated water
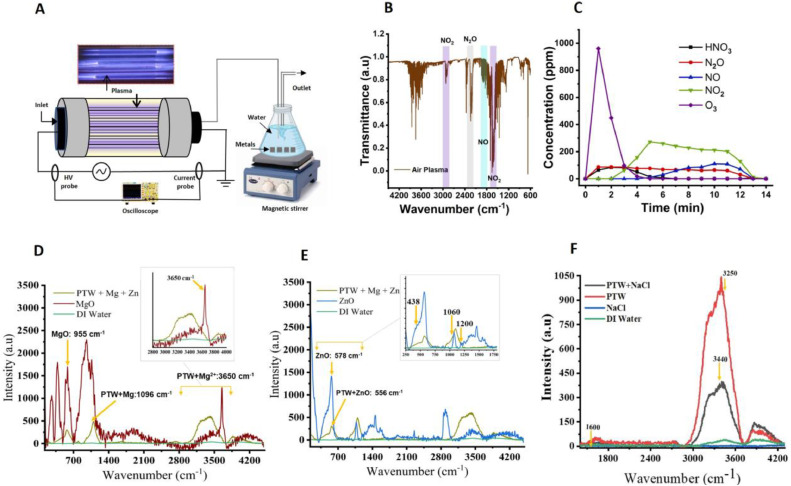
The electrical setup for ME-CDBD has been presented in Fig. 2A. The electrical properties and optical emission spectroscopy data of ME-CDBD plasma have already been reported in our previous study [31]. Here, we investigated the RONS generated in ME-CDBD plasma using gas phase FTIR spectroscopy (Fig. 2B), which revealed the presence of NO2 (1,540–1,660 and 2,840–2,940 cm−1), NO (1,700–2,000 cm−1), N2O (2,160–2,260 cm−1), and HNO3 (950–850 cm−1) (Fig. 2B). During the first 4 min, ozone gas was generated in the ME-CDBD plasma, which was found to dissociate and decrease with increasing temperature of the plasma reactor as well as by its reaction with NOx. Consequently, NO, N2O, and NO2 concentrations reached the maximum levels, and they were measured to be 110, 87, and 272 ppm, respectively (Fig. 2C).
Fig. 2.
Schematic of plasma device with gas phase FTIR and Raman bond analysis in water. (A) Schematic representation of ME-CDBD plasma source, (B) FTIR identification of RNS in ME-CDBD plasma, (C) Gas phase ROS/RNS concentration, and Raman peaks of (D) MgO (E) ZnO, and (F) effect of NaCl addition in PTW.
In the present study, PTW + Mg + Zn, PTW, and PTW + NaCl were analyzed using Raman spectroscopy, and the presence of Mg2+, Zn2+ metal ions, and NaCl was detected. The Raman peaks for magnesium oxide (MgO) at below 1,500 cm−1 and the prominent peak at 1,096 cm−1 corresponded to the defect modes caused by out-of-plane vibrations. Interestingly, unique interactions between the water molecules and Mg2+ were observed at 3,650 cm−1 (Fig. 2D). Similarly, Zn2+ oxidized at low temperature to form zinc oxide (ZnO) producing second-order Raman scattering at 438 cm−1, 578 cm−1, 1,060 cm−1 and 1,200 cm−1. Furthermore, ZnO in PTW gives rise to the peak 556 cm−1 (Fig, 2E). The number and energy of hydrogen bonds determined the bonding strength in the PTW treatment, which can be characterized by the O–H stretching vibration modes observed at 3,473 cm−1 (Fig, 2F). Thus, water molecules exhibited one O–H bending mode at 1,600 cm−1 and two O—H stretching modes with a broad spectral band at around 2,900–3,700 cm−1 (Fig, 2F). PTW exhibited enhanced hydrogen bond strength compared to DI. Through this phenomenon, water molecules enhanced the high surface tension, enabling PTW to preserve plasma-generated RNS such as nitrate (NO3−) and nitrite (NO2−) for a longer time (Fig, 2F). It is known that preserving short-lifetime species such as hydroxyl radicals (OH−), nitric oxide (NO), and peroxynitrite (ONOO−) is a challenge in non-thermal plasma discharge. However, long-lifetime species such as hydrogen peroxide (H2O2), nitrate (NO3−), and nitrite (NO2−) reactive species could be preserved using conventional techniques [45]. In general, NaCl is known to lack Raman peaks due to its dissociation from Raman-inactive mono-atomic two ions of Na+ and Cl- in water. However, their presence can be investigated indirectly by the presence of cations Na+ and anions Cl- in water. This changed the position and intensity of the Raman spectrum, where the O—H stretching bond intensity decreased at 3,250 and 3,440 cm−1 (Fig, 2F). Furthermore, H2O2, NOx, NO2, NO3, pH, electrical conductivity, TDS, and ORP of the irrigated water were measured, and the results are summarized in Fig. S1.
3.2. Growth improvement in Pak Choi plants under salinity stress
Photographs of the harvested Pak Choi plants under all treatment conditions on days 3, 6, and 9 are shown in Fig. 3A, B, and C, respectively. Plant growth showed a clear reduction under the influence of 100 mM NaCl concentration. Notably, the application of PTW and PTW + M resulted in improved Pak Choi seedlings with sequential increasing the treatment days compared with salinity stress alone, (Fig. 3D–I).
Fig. 3.
Improved Pak Choi growth under salinity. Photographs with top, side, and post-harvest view on (A) Day 3, (B) Day 6, and (C) Day 9. Graphs representing shoot length, (E) root length, (F) shoot fresh weight, (G) root fresh weight, (H) shoot dry weight, and (I) root dry weight. Student's t-test was used for statistical analysis with respect to DI (green colors) and NaCl (black colors), where p-value was denoted by ap < 0.05, bp < 0.01, and cp < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The obtained results on day 9 indicate that the irrigation of PTW and PTW+M significantly improved the plant growth parameters. Following irrigation treatments of PTW and PTW + M, the shoot length increased by 15.1% and 18.1%, compared to the control group, respectively. Similarly, root length showed 16.09% and 26.01% improvement. In the context of plant biomass, plant fresh weight increased 34.3% and 38.1% (in shoot), 17.6% and 31.7% (in root) following the irrigation treatments of PTW and PTW+M compared to control, respectively. Similarly, the plant dry weight showed 50.14% and 54.4% (in shoot), and 28.06% and 35.08% (in root) increase in treated groups compared to the control.
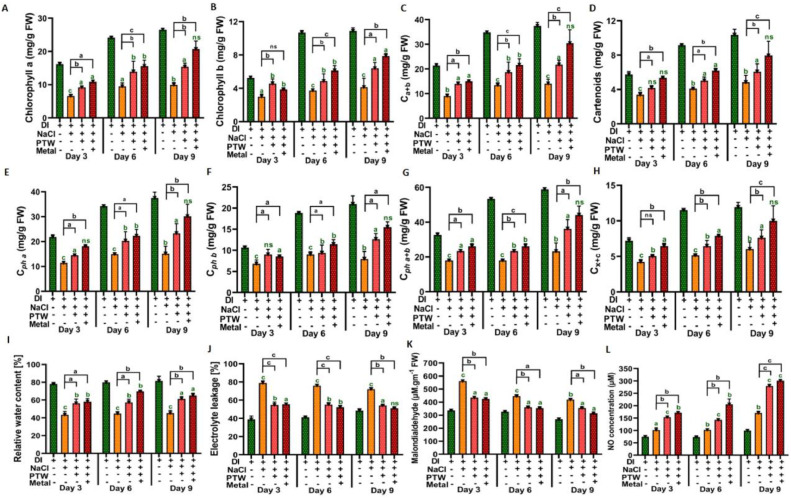
3.3. Determination of the chloroplast content, RWC, EL, lipid peroxidation, and nitric oxide concentration of the leaves
Chlorophyll a, chlorophyll b, total chlorophyll Ca+b, and carotenoid content are important in light harvesting. In addition, pheophytin a, pheophytin b, and total pheophytin, along with carotenoids are important in the electron transport chain. The obtained results indicate that the chlorophyll content, pheophytin, and carotenoids significantly increased after PTW and PTW + M irrigations compared to control groups (irrigated with DI water). The chlorophyll content improved 1.54 and 2.08 times after PTW and PTW + M treatment compared to the control group. Similarly, chlorophyll b showed 1.54 and 1.90-times improvements in treated groups (at day 9). The total chlorophyll content showed a 1.54-fold and 2.16-fold increase in PTW and PTW + M treated groups compared to the control (Fig. 4A–D). The pheophytin content improved 1.53 and 1.99 times after PTW and PTW + M treatment compared to the control group. Similarly, the pheophytin b showed 1.58 and 1.95-time improvements in treated groups (at day 9). The total pheophytin content showed a 1.55-fold and 1.89-fold increase in PTW and PTW + M treated groups compared to the control (Fig. 4E–H). Additionally, the water uptake ability in terms of RWC also significantly increased 1.35 and 1.44 times after PTW and PTW + M treatment compared to the control group (Fig. 4I). However, the content of EL significantly reduced by 35.56% and 44.35% after PTW and PTW + M treatment compared to the control group (at day 9), where an efflux of potassium (K+) along an influx of sodium (Na+) and chloride (Cl-) ions occurred. Consequently, MDA levels also decreased 16.12% and 26.06% after PTW and PTW+M treatment compared to the control group as shown in Fig. 4J–K. Furthermore, the levels of NO significantly increased by 64.01% and 76.46% in PTW and PTW + M treated groups compared to the control on day 9 (Fig. 4L). Thus, PTW and PTW + M improved the hydration capacity and ability to mitigate oxidative damage in Pak Choi which led to a high degree of improvement in the growth under salinity stress.
Fig. 4.
Content of (A) chlorophyll a, (B) chlorophyll b, (C) chlorophyll a + b, (D) total carotenoids, (E) pheophytins a, (F) pheophytins b, (G) pheophytin a + b, (H) total carotenoids, (I) relative water content, (J) electrolyte leakage, (K) malondialdehyde, and (L) NO concentration. The statistical analysis was done by the student t-test with respect to DI (green color) and NaCl (black color), where p-value was denoted by ap < 0.05, bp < 0.01, and cp < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
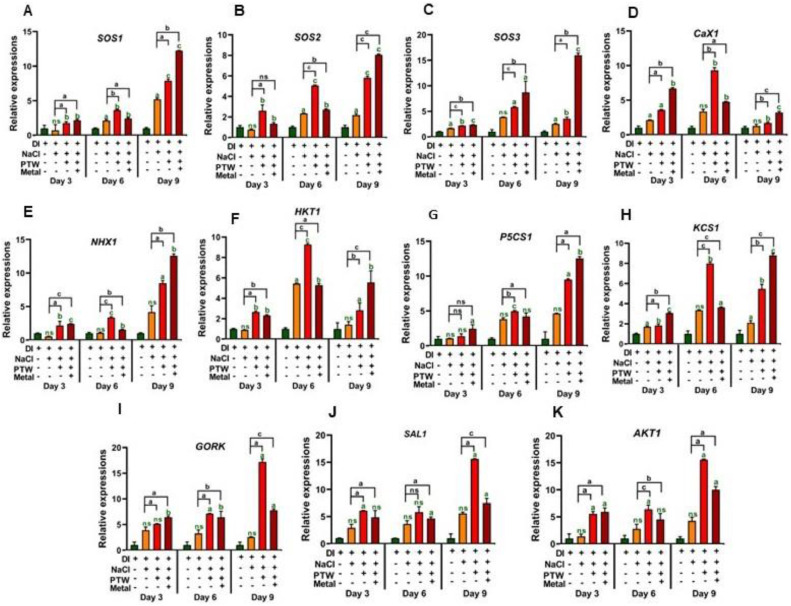
3.4. Molecular expression of SOS signaling pathway in Pak Choi plants
The SOS pathway is an important signaling mechanism for ionic homeostasis and regulating Na+ ion efflux from the cytoplasm, which is necessary to overcome salinity stress and enhance stress tolerance in plants. The obtained results indicate that the SOS complex, which has salt tolerance genes, significantly increased after PTW and PTW + M irrigations compared to control groups (irrigated with DI water). The gene expressions of SOS1 7.89 and 12.23 times, SOS2 5.81 and 8.05 times, and SOS3 5.81 and 8.05 times were upregulated after the PTW and PTW + M irrigation under salt stress on day 9, respectively as shown in Fig. 5A–C. Moreover, the genes associated with ionic balance, that is, CaX1 1.71 and 3.22 times, NHX1 8.48 and 12.58 times, and HKT1 2.83 and 5.55 times, which play crucial roles in the transport of Ca2+, Na+/H+, and K+, were upregulated by PTW and PTW + M irrigation under salinity stress, as shown in Fig. 5D–F. Additionally, osmo-protectant genes, that is, P5CS1 9.50 and 12.5 times, KCS1 5.46 and 8.80 times, GORK 17.2 and 7.78 times, SAL1 15.61 and 7.49 times, and AKT1 15.5 and 10.0 times were upregulated by PTW and PTW + M irrigation under salt stress, on day 9, respectively as shown in Fig. 5G–K. These coherent responses improved the regulatory influence of PTW and PTW + M on the reduction of salinity stress by triggering salinity tolerance and reestablishing redox and ionic homeostasis in Pak Choi seedlings.
Fig. 5.
Enhanced salt tolerance genes in Pak Choi Seedling by PTW and PTW + M under salinity stress. Expression of genes (A) SOS1, (B) SOS2, (C) SOS3, (D) CaX1, (E) NHX1, (F) HKT1, (G) P5CS1, (H) KCS1, (I) GORK, (J) SAL1 and (K) AKT1. The statistical analysis was done by the student t-test with respect to DI (green color), and NaCl (black color), where p-value was denoted by ap < 0.05, bp < 0.01, and cp < 0.001. Significance was calculated using both control groups (without and with salinity stress).
3.5. Detection of ROS, RNS, and calcium ions as redox balance in Pak Choi leaves under salinity stress
A histochemical technique was used to detect ROS, RNS, and Ca2+ ions in Pak Choi leaves. Confocal images of the adaxial surface of leaves were captured to trace ROS, RNS, and Ca2+ (Fig. 6A–C). Quantitative analysis of confocal images revealed the presence of elevated ROS levels on the adaxial surface of leaves under saline conditions. However, this effect was mitigated by PTW and PTW + M irrigation, as shown in Fig. 6A and D. In the control group, the RNS intensity is approximately 20%, a value that significantly escalates in both the PTW and PTW + M conditions, reaching approximately 32% and 50%, respectively, as shown in Fig. 6B and E. Notably, the PTW + M condition exhibits the maximum RNS levels in Pak Choi. This indicated that PTW and PTW + M served as sources of NO2-, NO3-, and predominantly NO, as shown in Fig. 6B and E. Similarly, Ca2+ levels increased substantially with PTW and PTW + M irrigation under saline conditions, as shown in Fig. 6C and F. Ultimately, the upregulation of intracellular RNS and Ca2+ levels by PTW and PTW + M irrigation contributed to the mitigation of salinity stress, thereby enhancing overall plant health.
Fig. 6.
The effect of PTW and PTW + M irrigation on intracellular RONS and Ca2+ levels in Pak Choi leaves under salinity stress. Visual representation of (A) ROS, (B) RNS, (C) Ca2+ in leaves and their quantitative representation as (D) ROS, (E) RNS and (F) Ca2+, respectively.
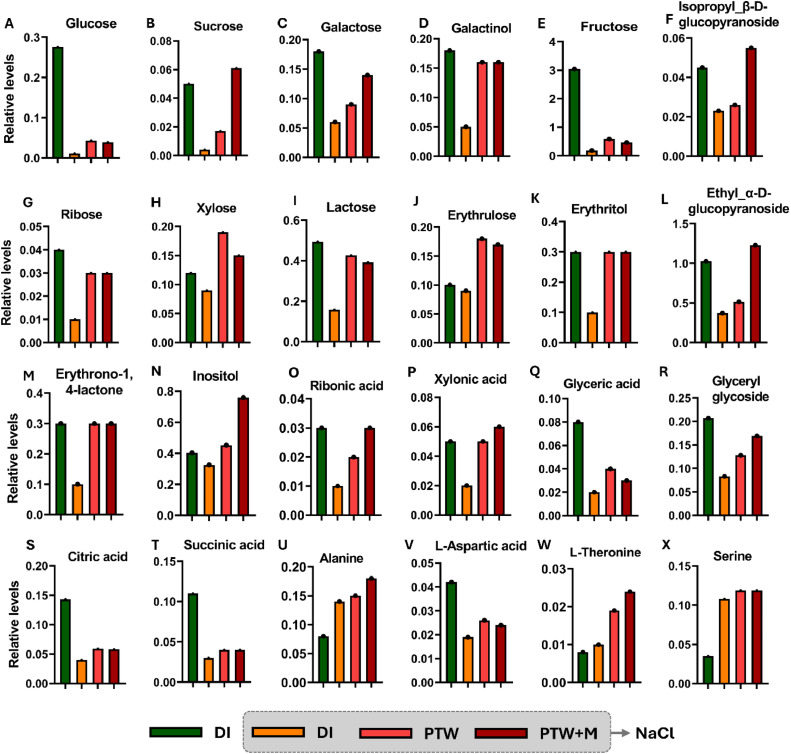
3.6. Upregulation of primary metabolites in Pak Choi seedlings by PTW and PTW + M irrigation under salinity stress
Primary metabolite alterations in glycolysis occur in the cytoplasm, and the tricarboxylic acid cycle in the mitochondria is linked to amino acids synthesis, which is essential for energy metabolism and adaptation [46]. The total ion chromatograms and typical chromatograms displayed peak capacities with respect to the relative abundance and reproducible retention time, indicating the reliability of the primary metabolites for each condition (Fig. S2). The accumulation of gluconate, which is involved in metabolism of sugars, such as glucose; 3.9-fold, 3.54-fold, sucrose; 4.25-fold, 15.25-fold, galactose; 1.5-fold, 2.33-fold, galactinol; 3.2-fold, 3.2-fold, fructose; 3.2-fold, 2.57-fold, isopropyl_β-D-glucopyranoside; 1.13-fold, 2.39-fold, ribose; 3-fold, 3-fold, xylose; 2.11-fold, 1.66-fold, lactose; 2.69-fold, 2.48-fold, and erythrulose; 2-fold, 1.88-fold, was upregulated by PTW and PTW + M irrigation under salinity stress (Fig. 7A–J). Sugar alcohols, i.e., erythritol; 3-fold, 3-fold, ethyl-α-D-glucopyranoside; 1.38-fold, 3.3-fold, erythrono-1,4-lactone; 3-fold, 3-fold, and inositol; 1.39-fold, 2.33-fold, were also upregulated by PTW and PTW + M irrigation under salinity stress (Fig. 7K–N). However, all sugars and sugar alcohols were downregulated under NaCl stress compared to DI. Moreover, organic acids, such as ribonic acid; 2-fold, 3-fold, xylonic acid; 2.5-fold, 3-fold, glyceric acid; 2-fold, 1.5-fold, glyceryl glycoside; 1.54-fold, 2.03-fold, citric acid; 1.47-fold, 1.45-fold, and succinic acid; 1.33-fold, 1.33-fold, and metabolic amino acids, such as alanine; 1-fold, 1.2-fold, L-aspartic acid; 1.36-fold, 1.26-fold, L-threonine; 2.4-fold, 1.9-fold, and serine; 1.1-fold, 1.1-fold, were upregulated by PTW and PTW + M irrigation under salinity stress (Fig. 7O–X). The upregulation of sugars, sugar alcohols, organic acids, and amino acids by PTW and PTW+M irrigation served as osmoprotectants in Pak Choi seedlings to mitigate salinity stress. The primary metabolites in Pak Choi seedlings with respect to their retention times are listed in Table S2.
Fig. 7.
Metabolite alternation involved in the primary pathway of Pak Choi leaves under salinity stress. Relative levels of sugars (A) Glucose (B) Sucrose, (C) Galactose, (D) Galactinol, (E) Fructose, (F) Isopropyl_β-D-glucopyranoside, (G) Ribose, (H) Xylose, (I) Lactose, (J) Erythrulose and sugar alcohols, (K) Erythritol, (L) Ethyl_α-D-glucopyranoside, (M) Erythrono-1,4-lactone, (N) Inositol and organic acids, (O) Ribonic acid, (P) Xylonic acid, (Q) Glyceric acid, (R) Glyceryl glycoside, (S) Citric acid, (T) Succinic acid and amino acids, (U) Alanine, (V) L-Aspartic acid, (W) L-Threonine and (X) Serine are detected in DI, NaCl, PTW, and PTW + M under salinity stress.
4. Discussion
The present study aimed to examine the effects of PTW and PTW + M irrigation on Pak Choi seedlings under salinity stress. When using atmospheric air comprising nitrogen 78% (N2) and oxygen 22% (O2) as the feed gas, the ME-CDBD process produces reactive nitrogen atoms that dissociate and form nitrogen oxide (NO), ozone (O3), and oxygen (O2) (Reaction 11) maintain a balance by interconverting between nitrogen dioxide (NO2) and NO (Reactions 12–15) [47]. While PTW promotes plant growth, its low pH could potentially inhibit the growth of plants sensitive to specific pH [48]. Mg2+ and Zn2+ metals could be added not only to neutralize the pH but also to react with NO2- and NO3− to produce magnesium and zinc nitrites and nitrates, which are essential nutrients for normal plant growth (Reactions 18–21) [49].
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
Additionally, the Mg2+ and Zn2+ are easily oxidized into MgO and ZnO at room temperature (Reactions 22–25); similarly Mg (OH)2 and Zn (OH)2 are formed during plasma treatment, which is confirmed in a second-order Raman scattering model (Fig. 2D, 2E) [50,51]. Interestingly, enhanced hydrogen bond strength was observed in PTW compared to that in DI because the O—H stretching vibration modes were observed at 3,473 cm−1. This phenomenon causes water molecules to have high surface tension enabling PTW to preserve plasma-generated RONS for an extended period [52]. NaCl lacks Raman-active modes owing to its dissociation property in water, that is, Na+ and Cl− (Fig. 2F).
| (22) |
| (23) |
| (24) |
| (25) |
The pH was significantly lower in PTW than in DI; however, it increased in NaCl due to its dissociation into Na+ and Cl− ions. The physicochemical properties of PTW include dominant RNS, that is, NO, NO2−, and NO3− and other ions contributing to the enhancement of ORP as the powerful oxidizing agent. This ultimately enhances the electrical conductivity (owing to the increasing concentration of charged particles) and TDS. However, a slight decrease in pH was observed in the PTW + M compared to that in PTW because of the presence of metals that remove the H+ ions by converting them into the nascent hydrogen [H] (Fig. S2A-S2H).
Abiotic stressors, mainly salinity stress, have drastically reduced crop production [53,54]. To enhance its agriculture yield, several methods, such as biofortification, agronomic practices and genetic engineering have been suggested. However, these methods can be complex and challenging to implement in practical agricultural settings. Recently, NAPP has emerged as a promising field in agriculture research because of the potential effects of PTW on seed germination and plant growth [26,55]. PTW is well known as a plant growth and immune inducer [56], although the efficiency varies depending on several factors, including plant species, the chemical composition of PTW, and experimental settings. PTW and PTW + M have been reported to have dominant RNS, such as NO, NO2−, and NO3−, which substitute for fertilizers during salinity stress [57]. Magnesium (Mg2+) and zinc (Zn2+) are also essential in mitigating the adverse effects of salinity stress on plant growth, physiology, and productivity. They attain this by upholding nutrient balance, and osmotic equilibrium, and by stimulating antioxidant defense mechanisms [[58], [59], [60]]. In the present study, the PTW and PTW + M treatments improved the Pak Choi seedling growth and biomass under salinity stress (Fig. 3A–I). However, PTW treatment has been also reported with similar results in wheat and barley seedlings under salinity stress [35,36].
Additionally, PTW and PTW + M also enhanced the plant chlorophyll and pheophytin, which are major pigments for photosynthesis. This positive effect of PTW has been reported on the growth of wheat (Triticum aestivum), which also showed similar results on the concentration of photosynthetic pigments [61]. Additionally, the signaling irrigation of PTW and PTW + M which has dominant RNS in the form of NO, NO2−, and NO3−, may improve the water-holding capacity of tissues by regulating the activity of aquaporins, which facilitates water movement across the cell membranes in Pak Choi seedlings under salinity stress. Consequently, the levels of MDA and EL decreased to maintain the membrane integrity and prevent the ion's leakage (Fig. 4A–L). Similar to our results melatonin and sodium nitroprusside (SNP) treatment has been reported to significantly reduce the increase of MDA and EL in tomato and rapeseed, respectively under stress [62,63].
In this work, PTW has dominant RNS involved in the modulation of SOS pathway genes, i.e., SOS1, SOS2, and SOS3 at the transcription level, which induces the cytosolic increase of calcium linked with SOS3 calcium-binding protein (Fig. 5A–C). However, ROS and RNS can also stimulate the SOS pathway to enhance salinity tolerance [64]. Likewise, Arabidopsis is correlated with the SOS pathway genes, which are associated with plasma membrane Na+/H+ exchanger, protein kinase, and a calcium-binding protein [65]. We found elevated expression of CaX1 after irrigation of PTW and PTW + M that encodes a vacuolar Ca2+/H+ antiporter that maintains the regulation of intracellular Ca2+ levels under salinity stress (Fig. 5D) [66]. Interestingly, NaCl induces extremely slight expression of CaX1 gene which proves that Ca2+ as secondary signal to maintain the cytoplasmic pH under salinity stress. NHX1 marker, which is associated with ionic homeostasis and pH regulation within plant cells under salinity stress (Fig. 5E) [67], HKT1 marker, which is associated with Na+ and K+ ions uptake under salinity stress (Fig. 5F) [68], P5CS1, which is associated with proline biosynthesis and osmolytes necessary to overcome the oxidative stress (Fig. 5G) [69], KCS1, which is associated with fatty acid biosynthesis and increase of apo-plastic barrier suberin deposition in response to salinity stress (Fig. 5H), [70], SAL1 gene, which is associated with ionic balance and regulation of the osmotic stress particularly under high salinity stress (Fig. 5G) [71], AKT1 marker, which is associated with K+ ions transport in plant cells, ensuring ionic balance under salinity stress (Fig. 5K) [72] all were significantly up-regulated in Pak choi leaves under PTW and PTW + M irrigation. The intracellular increase of NO and Ca2+ levels as a secondary signal was observed after irrigation of PTW and PTW + M under salinity stress (Fig. 6A–F). PTW has been reported in the modulation of cytosolic calcium in Arabidopsis thaliana plants [73].
It is well known that NO promotes the plants to survive under salinity stress by increasing the antioxidant defensive mechanism, osmolyte accumulation and ionic balance [74]. Similarly, we found that the irrigation of PTW and PTW + M improved the accumulation of primary metabolites, including gluconate, in which sugars, such as glucose, sucrose, galactose, galactinol, fructose, isopropyl_β-D-glucopyranoside, ribose, xylose, lactose, and erythrulose are increased (Fig. 7A–J). However, PTW also enhanced the salinity tolerance in barley by enhancing the carotenoid content specifically, β-carotene and xanthophyll [35,75]. Moreover, sugar alcohols, such as erythrono-1,4-lactone, Inositol, Isopropyl-β-D-glucopyranoside, and ethyl-α-D-glucopyranoside are increased along with increase in sugars contents, i.e., erythritol, ethyl-α-D-glucopyranoside, erythrono-1,4-lactone, inositol. Also, organic acids are found to be increased, in which ribonic acid, xylonic acid, glyceric acid, glyceryl glycoside, citric acid, and succinic acids, are included (Fig. 7K–O). It is reported that the PTW treatment enhanced the antioxidant activity such as phenolics, flavonoids, and ascorbic acid to mitigate the salinity stress [76]. Amino acids, such as serine, alanine, L-aspartic acid, and L-threonine are also increased in Pak Choi plants under salinity (Fig. 7P–X). Thus, carbohydrates were found to be involved in metabolic processes, such as glycolysis, tricarboxylic acid cycle or Krebs cycle, and pentose phosphate pathway or hexose monophosphate shunt, which were upregulated in salinity stress plants by PTW and PTW + M irrigating. Overall, primary metabolic components, such as erythrulose, xylose, inositol and serine, L-threonine, were all related to osmolyte and antioxidant defense systems, which were enhanced in PTW and PTW+M in comparison to that in DI.
NAPP priming could enhance the stress tolerance in tomato plants suggesting the role of RONS signaling in plant defense [56]. The effect of RONS as signaling molecules in barley and Arabidopsis was also discussed under the positive effects of salinity stress [35,77]. Based on our findings, we propose a mechanism involved in salt tolerance in Pak Choi seedlings under salinity by PTW and PTW + M irrigation (Fig. 8). PTW and PTW + M have mixed contents of mainly RNS such as NO, NO2- and NO3- along with Mg2+ and Zn2+ ions which upregulate the salt tolerance SOS complex genes and ionic and redox balance genes, that is, CaX1, NHX1, and HKT1. Moreover, the cell membrane potential genes, such as KCS1, GORK, SAL1, AKT1, ultimately improved plant growth and chlorophyll and pheophytin content by controlling MDA and EL. Additionally, PTW and PTW + M enhanced RWC, intracellular NO, and Ca2+and primary metabolites, which are important for mitigating salinity stress (Fig. 8). Thus, our results suggest that PTW could be utilized as a novel priming agent against plant environmental stresses. The safe doses of PTW mainly modulate the plant growth and immunity which triggers an adaptive response that mitigates the detrimental effects of stress.
Fig. 8.
Proposed molecular mechanism of salt tolerance in Pak Choi seedlings under salinity by PTW and PTW + M. The ionic homeostasis and osmotic adjustment genes were induced by the irrigations of PTW and PTW + M. The PTW and PTW + M which have dominant RNS, triggered the plantʼs various metabolic and physiological processes, which may involve in the stimulation of intracellular signaling molecules (Ca2+) and NO that interact with each other to provide the proper response to the stress tolerance.
5. Conclusion
To improve the plant tolerance to salinity, we investigated the effects of PTW and PTW + M on Pak Choi seedlings. Under salinity stress, PTW and PTW + M significantly improved plant growth, photosynthetic activity, cellular hydration, and NO and Ca2+ levels by activating the ionic balance and osmotic adjustment genes, namely SOS, CaX1, NHX1, HKT1, P5CS1, KCS1, GORK and SAL1. Moreover, the accumulation of primary metabolites, such as gluconate, organic acids, and amino acids, was enhanced by PTW and PTW + M, which improved the Osmo protectant ability and adaptations to mitigate salinity stress. Thus, PTW and PTW + M irrigation mitigated salinity stress by promoting osmotic adjustment, membrane impermeability, hydration capacity, and the upregulation of salt tolerance genes and primary metabolites in Pak Choi seedlings under salinity stress. Our results are key to understanding the role of PTW and PTW + M with the underlying mechanism to increase the plant tolerance against salinity stress. These findings might play a critical role in further investigations in this domain and effective implementation of NAPP technology to advance sustainable agriculture.
CRediT authorship contribution statement
Rida Javed: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. Sohail Mumtaz: Writing – original draft, Visualization, Formal analysis, Writing – review & editing. Kirubel Amsalu: Investigation, Writing – review & editing. Eun Ha Choi: Writing – review & editing, Supervision, Resources, Conceptualization, Funding acquisition.
Acknowledgments
Declaration of competing interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
This work was supported by the (NRF) with grants funded by the Korean government (MIST) (NRF-2021R1A6A1A03038785), by the MSIT (Ministry of Science and ICT), Korea, under the ITRC (Information Technology Research Center) support program (IITP-2020-0-01846), and partially by Kwangwoon University in 2024.
Biographies
Rida Javed holds a master’s degree in Botany, awarded in 2020, from University of Agriculture, Faisalabad Pakistan. Currently, she is pursuing her Ph. D. at Kwangwoon University, Seoul, South Korea. Her research focused on the agricultural application of Plasma treated water in plant abiotic stress mainly salinity. Furthermore, her research also focused on application of plasma technology to plant growth and development exploring its molecular mechanism underlying positive effects of plasma.
Eun Ha Choi is a renowned figure in the fields of plasma medicine and pulsed power technology for HPM generation within the realm of plasma physics. Currently serving as the director of the Plasma Bioscience Research Center/Applied Plasma Medicine Center at Kwangwoon University in Seoul, Korea. He has also worked as professor and senior researcher positions at Texas Tech University (2001–2003) and Hampton University/NASA (1989–1990) and Naval Surface Warfare Center (NSWC) at MD, USA (1987–1989) in the field of plasma physics. Especially, his works on plasma medicine are focused on revolutionary breakthrough via plasma interactions with biological tissues/cells.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fmre.2024.06.010.
Appendix. Supplementary materials
References
- 1.Tester M., Langridge P. Breeding technologies to increase crop production in a changing world. Science. 2010;327(80):818–822. doi: 10.1126/science.1183700. [DOI] [PubMed] [Google Scholar]
- 2.Iqbal B., Zhao X., Khan K.Y., et al. Microplastics meet invasive plants: Unraveling the ecological hazards to agroecosystems. Sci. Total Environ. 2024;906 doi: 10.1016/j.scitotenv.2023.167756. [DOI] [PubMed] [Google Scholar]
- 3.Deinlein U., Stephan A.B., Horie T., et al. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19:371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ke Q., Wang Z., Ji C.Y., et al. Transgenic poplar expressing codA exhibits enhanced growth and abiotic stress tolerance. Plant Physiol. Biochem. 2016;100:75–84. doi: 10.1016/j.plaphy.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Khan A.R., Ulhassan Z., Li G., et al. Micro/nanoplastics: Critical review of their impacts on plants, interactions with other contaminants (antibiotics, heavy metals, and polycyclic aromatic hydrocarbons), and management strategies. Sci. Total Environ. 2023;912:169420. doi: 10.1016/j.scitotenv.2023.169420. [DOI] [PubMed] [Google Scholar]
- 6.Ji H., Pardo J.M., Batelli G., et al. The salt overly sensitive (SOS) pathway: Established and emerging roles. Mol. Plant. 2013;6:275–286. doi: 10.1093/mp/sst017. [DOI] [PubMed] [Google Scholar]
- 7.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J.-K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y., Guo Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018;60:796–804. doi: 10.1111/jipb.12689. [DOI] [PubMed] [Google Scholar]
- 10.Van Zelm E., Zhang Y., Testerink C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020;71:403–433. doi: 10.1146/annurev-arplant-050718-100005. [DOI] [PubMed] [Google Scholar]
- 11.Chen M., Yang Z., Liu J., et al. Adaptation mechanism of salt excluders under saline conditions and its applications. Int. J. Mol. Sci. 2018;19:3668. doi: 10.3390/ijms19113668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J.-K., Liu J., Xiong L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell. 1998;10:1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Q.-S., Guo Y., Quintero F.J., et al. Regulation of vacuolar Na+/H+ exchange in arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway*. J. Biol. Chem. 2004;279:207–215. doi: 10.1074/jbc.M307982200. [DOI] [PubMed] [Google Scholar]
- 14.Venema K., Quintero F.J., Pardo J.M., et al. The Arabidopsis Na+/H+Exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes*. J. Biol. Chem. 2002;277:2413–2418. doi: 10.1074/jbc.M105043200. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui M.H., Al-Whaibi M.H., Basalah M.O. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma. 2011;248:447–455. doi: 10.1007/s00709-010-0206-9. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y., He J., Liu H., et al. Nitric oxide alleviates deterioration and preserves antioxidant properties in ‘Tainong'mango fruit during ripening. Hortic. Environ. Biotechnol. 2017;58:27–37. [Google Scholar]
- 17.Lau S.E., Hamdan M.F., Pua T.L., et al. Plant nitric oxide signaling under drought stress. Plants. 2021;10(360):360. doi: 10.3390/plants10020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zulfiqar F., Ashraf M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021;160:257–268. doi: 10.1016/j.plaphy.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Franzoni G., Cocetta G., Trivellini A., et al. Effect of exogenous application of salt stress and glutamic acid on lettuce (Lactuca sativa L. Sci. Hortic. (Amsterdam). 2022;299 [Google Scholar]
- 20.Iqbal B., Khan I., Anwar S., et al. Biochar and saline soil: Mitigation strategy by incapacitating the ecological threats to agricultural land. Int. J. Phytoremed. 2024;26:1–11. doi: 10.1080/15226514.2024.2310001. [DOI] [PubMed] [Google Scholar]
- 21.Tariq M., Iqbal B., Khan I., et al. Microplastic contamination in the agricultural soil—Mitigation strategies, heavy metals contamination, and impact on human health: A review. Plant Cell Rep. 2024;43:1–15. doi: 10.1007/s00299-024-03162-6. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal B. Microplastics and invasive alien plants: A change in soil ecology deliberately impacts the aboveground productivity of the crops. J. Soil Plant Environ. 2024;3:1–7. [Google Scholar]
- 23.Mumtaz S., Rana J.N., Lim J.S., et al. Effect of plasma on-time with a fixed duty ratio on reactive species in plasma-treated medium and its significance in biological applications. Int. J. Mol. Sci. 2023;24:5289. doi: 10.3390/ijms24065289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priatama R.A., Pervitasari A.N., Park S., et al. Current advancements in the molecular mechanism of plasma treatment for seed germination and plant growth. Int. J. Mol. Sci. 2022;23:4609. doi: 10.3390/ijms23094609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perea-Brenes A., Garcia J.L., Cantos M., et al. Germination and first stages of growth in drought, salinity, and cold stress conditions of plasma-treated barley seeds. ACS Agric. Sci. Technol. 2023;3:760–770. doi: 10.1021/acsagscitech.3c00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ka D.H., Priatama R.A., Park J.Y., et al. Plasma-activated water modulates root hair cell density via root developmental genes in Arabidopsis thaliana L. Appl. Sci. 2021;11:2240. [Google Scholar]
- 27.Mumtaz S., Javed R., Rana J.N., et al. Pulsed high power microwave seeds priming modulates germination, growth, redox homeostasis, and hormonal shifts in barley for improved seedling growth: Unleashing the molecular dynamics. Free Radic. Biol. Med. 2024;222:371–385. doi: 10.1016/j.freeradbiomed.2024.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Mumtaz S., Khan R., Rana J.N., et al. Review on the biomedical and environmental applications of nonthermal review on the biomedical and environmental applications of nonthermal plasma. Catalysts. 2023;13:685. [Google Scholar]
- 29.Ndiffo Yemeli G.B., Švubová R., Kostolani D., et al. The effect of water activated by nonthermal air plasma on the growth of farm plants: Case of maize and barley. Plasma Process. Polym. 2021;18 [Google Scholar]
- 30.Song J.-S., Kim S.B., Ryu S., et al. Emerging plasma technology that alleviates crop stress during the early growth stages of plants: A review. Front. Plant Sci. 2020;11:988. doi: 10.3389/fpls.2020.00988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puač N., Gherardi M., Shiratani M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018;15 [Google Scholar]
- 32.Bradu C., Kutasi K., Magureanu M., et al. Reactive nitrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J. Phys. D. Appl. Phys. 2020;53 [Google Scholar]
- 33.Misra N.N., Schlüter O., Cullen P.J. In: Cold Plasma in Food and Agriculture. Elsevier; 2016. Plasma in food and agriculture; pp. 1–16. [Google Scholar]
- 34.Zhou R., Li J., Zhou R., et al. Atmospheric-pressure plasma treated water for seed germination and seedling growth of mung bean and its sterilization effect on mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019;53:36–44. [Google Scholar]
- 35.Gierczik K., Vukušić T., Kovács L., et al. Plasma-activated water to improve the stress tolerance of barley. Plasma Process. Polym. 2020;17 [Google Scholar]
- 36.Iranbakhsh A., Ardebili N.O., Ardebili Z.O., et al. Non-thermal plasma induced expression of heat shock factor A4A and improved wheat (Triticum aestivum L.) growth and resistance against salt stress. Plasma Chem. Plasma Process. 2018;38:29–44. [Google Scholar]
- 37.Liang T., Ding H., Wang G., et al. Sulfur decreases cadmium translocation and enhances cadmium tolerance by promoting sulfur assimilation and glutathione metabolism in Brassica chinensis L. Ecotoxicol. Environ. Saf. 2016;124:129–137. doi: 10.1016/j.ecoenv.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Javed R., Mumtaz S., Choi E.H., et al. Effect of plasma-treated water with magnesium and zinc on growth of Chinese cabbage. Int. J. Mol. Sci. 2023;24:8426. doi: 10.3390/ijms24098426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lichtenthaler H.K. Chlorophylls and caroteniods pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:183–350. [Google Scholar]
- 40.Sumanta N., Haque C.I., Nishika J., et al. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014;2231:606X. [Google Scholar]
- 41.Ahmad S., Kamran M., Ding R., et al. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ. 2019;7:e7793. doi: 10.7717/peerj.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu W., Zhang Q., Ervin E.H., et al. Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front. Plant Sci. 2017;8:1017. doi: 10.3389/fpls.2017.01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Xu L., Shen H., et al. Metabolomic analysis with GC-MS to reveal potential metabolites and biological pathways involved in Pb & Cd stress response of radish roots. Sci. Rep. 2015;5:18296. doi: 10.1038/srep18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsoukou E., Bourke P., Boehm D. Temperature stability and effectiveness of plasma-activated liquids over an 18 months period. Water. 2020;12:3021. [Google Scholar]
- 46.Bandehagh A., Taylor N.L. Can alternative metabolic pathways and shunts overcome salinity induced inhibition of central carbon metabolism in crops? Front. Plant Sci. 2020;11:1072. doi: 10.3389/fpls.2020.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matějka F., Galář P., Khun J., et al. Mechanisms leading to plasma activated water high in nitrogen oxides. Phys. Scr. 2023;98:45619. [Google Scholar]
- 48.Lamichhane P., Veerana M., Lim J.S., et al. Low-temperature plasma-assisted nitrogen fixation for corn plant growth and development. Int. J. Mol. Sci. 2021;22:5360. doi: 10.3390/ijms22105360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamichhane P., Paneru R., Nguyen L.N., et al. Plasma-assisted nitrogen fixation in water with various metals. React. Chem. Eng. 2020;5:2053–2057. [Google Scholar]
- 50.Sharma A., Singh B.P., Dhar S., et al. Effect of surface groups on the luminescence property of ZnO nanoparticles synthesized by sol–gel route. Surf. Sci. 2012;606:L13–L17. [Google Scholar]
- 51.Gordeeva A., Hsu Y.-J., Jenei I.Z., et al. Layered zinc hydroxide dihydrate, Zn5(OH)10·2H2O, from hydrothermal conversion of ε-Zn(OH)2 at gigapascal pressures and its transformation to nanocrystalline ZnO. ACS Omega. 2020;5:17617–17627. doi: 10.1021/acsomega.0c02075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huš M., Urbic T. Strength of hydrogen bonds of water depends on local environment. J. Chem. Phys. 2012;136:144305. doi: 10.1063/1.3701616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jampeetong A., Brix H. Effects of NaCl salinity on growth, morphology, photosynthesis and proline accumulation of Salvinia natans. Aquat. Bot. 2009;91:181–186. [Google Scholar]
- 54.Passioura J.B. Scaling up: The essence of effective agricultural research. Funct. Plant Biol. 2010;37:585–591. [Google Scholar]
- 55.Vichiansan N., Chatmaniwat K., Sungkorn M., et al. Effect of plasma-activated water generated using plasma jet on tomato (Solanum lycopersicum L. var. cerasiforme) seedling growth. J. Plant Growth Regul. 2023;42:935–945. [Google Scholar]
- 56.Adhikari B., Adhikari M., Ghimire B., et al. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum) Free Radic. Biol. Med. 2020;156:57–69. doi: 10.1016/j.freeradbiomed.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Stoleru V., Burlica R., Mihalache G., et al. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-77355-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cakmak I., Kirkby E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008;133:692–704. doi: 10.1111/j.1399-3054.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- 59.Grotz N., Lou Guerinot M. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006;1763:595–608. doi: 10.1016/j.bbamcr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Shao J., Tang W., Huang K., et al. How does zinc improve salinity tolerance? Mechanisms and future prospects. Plants. 2023;12:3207. doi: 10.3390/plants12183207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kučerová K., Henselová M., Slováková Ľ., et al. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Processes Polym. 2019;16(3):1800131. [Google Scholar]
- 62.Hasan M.K., Ahammed G.J., Yin L., et al. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015;6:601. doi: 10.3389/fpls.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao G., Zhao Y., Yu X., et al. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 2018;19:1912. doi: 10.3390/ijms19071912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amir R., Munir F., Kubra G., et al. Role of signaling pathways in improving salt stress in plants. Salt Stress. Microbes, Plant Interact. Mech. Mol. Approach. 2019;2:183–211. [Google Scholar]
- 65.Rolly N.K., Imran Q.M., Lee I.-J., et al. Salinity stress-mediated suppression of expression of salt overly sensitive signaling pathway genes suggests negative regulation by AtbZIP62 transcription factor in Arabidopsis thaliana. Int. J. Mol. Sci. 2020;21:1726. doi: 10.3390/ijms21051726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Catalá R., Santos E., Alonso J.M., et al. Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell. 2003;15:2940–2951. doi: 10.1105/tpc.015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheikh-Mohamadi M.-H., Etemadi N., Aalifar M., et al. Salt stress triggers augmented levels of Na+, K+ and ROS alters salt-related gene expression in leaves and roots of tall wheatgrass (Agropyron elongatum) Plant Physiol. Biochem. 2022;183:9–22. doi: 10.1016/j.plaphy.2022.04.022. [DOI] [PubMed] [Google Scholar]
- 68.Ali A., Maggio A., Bressan R.A., et al. Role and functional differences of HKT1-type transporters in plants under salt stress. Int. J. Mol. Sci. 2019;20:1059. doi: 10.3390/ijms20051059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Funck D., Baumgarten L., Stift M., et al. Differential contribution of P5CS isoforms to stress tolerance in Arabidopsis. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.565134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krishnamurthy P., Ranathunge K., Franke R., et al. The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.) Planta. 2009;230:119–134. doi: 10.1007/s00425-009-0930-6. [DOI] [PubMed] [Google Scholar]
- 71.Quintero F.J., Garciadeblas B., Rodríguez-Navarro A. The SAL1 gene of Arabidopsis, encoding an enzyme with 3’(2’), 5’-bisphosphate nucleotidase and inositol polyphosphate 1-phosphatase activities, increases salt tolerance in yeast. Plant Cell. 1996;8:529–537. doi: 10.1105/tpc.8.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C.-F., Han G.-L., Yang Z.-R., et al. Plant salinity sensors: Current understanding and future directions. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.859224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortese E., Settimi A.G., Pettenuzzo S., et al. Plasma-activated water triggers rapid and sustained cytosolic Ca2+ elevations in Arabidopsis thaliana. Plants. 2021;10:2516. doi: 10.3390/plants10112516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan M.N., Siddiqui M.H., Mohammad F., et al. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide. 2012;27:210–218. doi: 10.1016/j.niox.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Peng R., Li X., Lou J., et al. Regulation of the soil microbial metabolism through alterations in the vegetative community in wetlands. Pol. J. Environ. Stud. 2023;32:1. [Google Scholar]
- 76.Hsu S.-C., Kong T.-K., Chen C.-Y., et al. Plasma-activated water affects the antioxidant contents in water spinach. Appl. Sci. 2023;13:3341. [Google Scholar]
- 77.Bafoil M., Le Ru A., Merbahi N., et al. New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses. Sci. Rep. 2019;9:8649. doi: 10.1038/s41598-019-44927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.