Abstract
The health economic value of insulin is usually expressed within a cost‐effectiveness framework providing an estimated incremental cost per quality‐adjusted life year (QALY) gained. Insulin clinical trials adopt a treat‐to‐target design in which both intervention and control arms aim to achieve similar levels of glycaemic control thereby allowing a comparison of secondary safety outcomes such as hypoglycaemia and weight gain. While of use to inform clinicians about the new insulin's tolerability, it is of limited use for an economic evaluation. An insulin's true potential value requires an assessment of the relationship between the benefits of attaining individualised glycaemic goals versus the factors known to act as barriers to the initiation/intensification of insulin and that also contribute to poor adherence in clinical practice. Addressing the rising demands that diabetes will impose upon the healthcare system will require the simultaneous execution of multiple strategies that acknowledge population dynamics, healthcare delivery constraints, the role of innovation and funding requirements. Accounting for patient‐specific characteristics to develop individualised plans and utilising technologies that address relevant barriers to care will require a whole‐system perspective on healthcare value and an appreciation of the interconnectivity of stakeholder needs. Importantly, convenience and treatment satisfaction are often not considered valuable features of insulin therapy; not only do they have value, but they are essential to addressing rising demands.
Plain Language Summary
More people around the world are living with diabetes. This is because people are living longer, populations are getting older, and more people are developing the disease. Clinicians will have to prescribe insulin for more people. To make well‐informed decisions about how to spend money on diabetes care, we need to understand how much therapies costs and how well they work. In healthcare, people often talk about “value for money.” This means getting better results without spending more money, or saving money without making things worse.
However, it's not always easy to figure out the value of new types of insulin. The way insulin is studied in clinical trials doesn’t always relate well to how it works, and is used, in real‐life clinical practice. Many studies don’t look at all the things that matter, like how easy it is for people to use the insulin or how it affects their daily lives.
When two types of insulin have comparable efficacy in terms of lowering blood sugar, other things — like side effects, how easy it is to use, and how well people stick to their treatment — become important drivers of value. These things are different for each person, may be left out of studies and may not considered to be important by decision makers.
In this paper, we first discuss how the health economics of insulin has traditionally been studied, and look at the findings, advantages and disadvantages of these approaches. We also describe how tools like continuous glucose monitors (which track blood sugar all the time) can help people improve outcomes.
We introduce what we call the “insulin value system.” This looks at how the features of the insulin, the patient's circumstances, and the state of the healthcare system interact to determine the value of a new therapy. In the case of insulin therapies, traditional approaches to value assessment don’t always capture the full picture.
Our second goal is to talk about the big challenges in diabetes care, for which there are no easy solutions. More people are getting diabetes, and more money will be needed for treatment and disease management. We believe that to really help make a difference, we need to consider the whole healthcare system, how everything is connected, and not just focus on one part. We believe it's important to look at how people live and work, their personal circumstances and how they and the healthcare system interact when assessing the value of insulin. That way, we can better understand how new treatments can help both people and the wider healthcare system.
Keywords: cost‐effectiveness, health economics, insulin therapy, pharmaco‐economics
1. INTRODUCTION
Investment in adequate health and social care is a prerequisite to maximising individual health and national economic prosperity. The magnitude of this investment globally is estimated at $8.3 trillion, or 10% of global gross domestic product. 1 It is unsurprising, therefore, that decision‐making within healthcare is closely aligned with the concept of maximising value for money. In 2010, Porter defined value as the health outcomes achieved that matter to patients relative to the cost of achieving those outcomes. 2 Therefore, improving value is achieved by either attaining better outcomes at no additional cost or saving money whilst maintaining standards. As such, efforts to improve patient outcomes relative to the cost of care reflect an aspirational goal shared across healthcare stakeholders.
Economic evaluations in healthcare are designed to inform decision‐making regarding the efficient allocation of limited resources and involve a comparative analysis of alternative courses of action designed to capture both costs and consequences. 3 These alternative courses of action could be the introduction of a new health procedure, a specialist‐led service or an educational programme. In each, the alternative is usual care. Economic evaluations are a core feature of health technology assessments (HTA); the alternative course of action typically being the evaluation of a new pharmaceutical product compared to existing therapies. Cost‐effectiveness analysis (CEA) is commonly used to estimate the incremental costs and health benefits associated with the new technology. 3 Value is often expressed as the incremental cost per quality‐adjusted life year (QALY) gained and compared to an established willingness‐to‐pay threshold. Quite apart from issues regarding affordability, most HTAs have limited consideration of the downstream or upstream implications on healthcare service delivery associated with the new technology. Consequently, value from the HTA perspective may not accurately capture the breadth of potential benefit at the healthcare system level. In recognition that conventional approaches to HTA may omit important drivers of value ISPOR, the professional society for health economics and outcomes research globally, published a value flower in 2018. 4 The value elements (petals) of the flower expand the usual cost and QALY metrics to consider a broader set of domains including insurance value, value of hope, value of knowing and equity amongst others. It is noteworthy that these additional value metrics are still considered within the relatively narrow context of a comparative analysis, conducted at a single point in the clinical pathway and undertaken at the patient or cohort level.
Decisions that optimise value from a single‐stakeholder perspective, such as the payer, may have sub‐optimal or unintended consequences elsewhere in the system. This is known as bounded rationality, where reasonable decisions are made using information from part of the system without considering the consequences elsewhere in the system. 5 Therefore, the answer to the question ‘Will this decision deliver a positive value outcome’? is inevitable: it depends! It depends on the state of the healthcare system at a particular point in time, how the elements within the system are affected, how they interact, what relevant constraints exist and how circumstances might change over time. The purpose of the system is straightforward: to improve health and well‐being. Yet the interaction between system elements is both complex and dynamic; consequently, aligning decision‐making to a common goal is challenging but not impossible.
There are currently an estimated 4.6 million people in the UK diagnosed with diabetes. 6 Insulin therapy is a necessity for patients with type 1 diabetes (T1D) and is typically initiated in type 2 diabetes (T2D) in those with sub‐optimal glycaemic control despite lifestyle modifications and non‐insulin treatment. 7 Observational data from the UK indicate that 11.7% of adults with T2D are prescribed insulin. 8 Achieving optimal glycaemic control reduces the risk of long‐term micro‐ and macro‐vascular complications, thereby improving the length and quality of life for people with diabetes and reducing healthcare resource utilisation and cost. The price of insulin reflects a number of factors, including its complex manufacturing process, the limited number of manufacturers, supply chain mark‐ups, and national policies for determining price. 9 The affordability of insulin has received notable attention in recent years, and given the clinical significance of insulin, the objective of this paper is to first characterise the health economics of insulin therapy and then discuss the more challenging aspects of addressing the rising demands in diabetes and barriers to achieving optimal outcomes.
2. THE HEALTH ECONOMICS OF INSULIN THERAPY
The aetiology of T1D and T2D differs, the former caused by the immune‐mediated destruction of the pancreatic β‐cells; the latter caused by decreased insulin action due to impaired insulin secretion and insulin resistance. Despite the differences in aetiology across diabetes types, common features arise with disease progression. For example, in T2D, pancreatic β‐cell failure may eventually occur, while in T1D, insulin‐induced weight gain, a sedentary lifestyle, and a high‐calorie diet can induce insulin resistance and increase cardiovascular risk. 10
The demand for insulin will undoubtedly grow over time. Previous studies have estimated the worldwide use of insulin in T2D will likely increase by 20% between 2018 and 2030, driven by a combination of increasing disease incidence, longer life expectancy and ageing populations. 11 Consequently, understanding the economics of insulin therapy in T1D and T2D is essential to rationalise its long‐term affordability, particularly within the context of emerging innovations in insulin pharmacology.
These innovations include ultra‐rapid‐acting insulin, providing increased control of postprandial glucose excursions without increasing hypoglycaemia risk, and ultra‐long‐acting insulin providing dosing flexibility with low risk of hypoglycaemia; 2nd and 3rd generation recombinant human DNA analogues, inhaled insulin, and potentially oral and smart insulin. 12 The availability of a weekly basal insulin offers the benefit of fewer injections and a likely improvement to therapy adherence; it may also contribute to addressing barriers to insulin initiation in type 2 diabetes. 13 Such innovations will undoubtedly exert further economic pressure upon payers seeking to reconcile cost and health benefit.
Assessing the value of new diabetes technologies typically utilises registrational clinical trial data characterising changes in levels of glycaemic control, body weight, blood pressure, cholesterol, renal function and the frequency of safety outcomes such as hypoglycaemia. The use of established risk equations 14 , 15 to estimate the expected impact on long‐term clinical outcomes associated with changes in these modifiable risk factors is routine. More recently, several cardiovascular outcomes trials have provided direct evidence of an effect on clinical outcomes 16 allowing the development of trial‐specific event equations to be developed and utilised within diabetes models. 17 Such evidence, combined with risk factor trajectories and therapy intensification algorithms, is used to estimate the expected long‐term (typically lifetime) clinical outcomes, costs, life expectancy and quality‐adjusted life expectancy associated with a new technology, as illustrated in Figure 1. For insulin, evidence of comparative effectiveness commonly utilises clinical trial data characterising differences in rates of hypoglycaemia, changes in body mass index and glycated haemoglobin (HbA1c). In T1D, basal/bolus insulin regimens are typically evaluated, while T2D regimens normally consider basal insulin combined with oral therapies or basal/bolus regimens. 18 , 19
FIGURE 1.
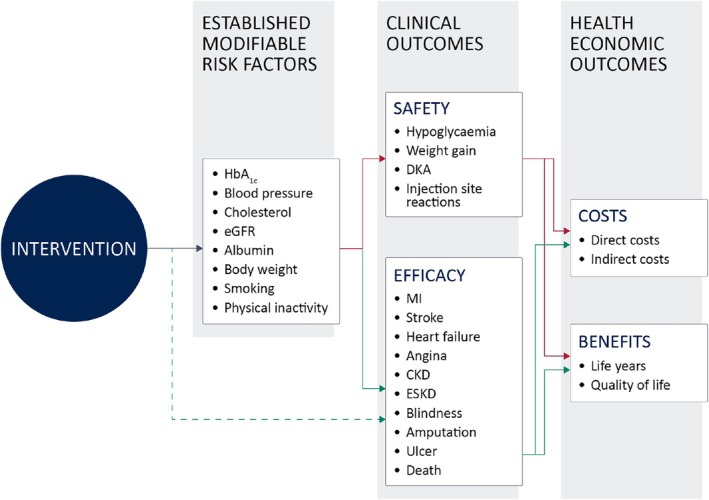
The standard mechanics of predicting future costs and health outcomes in diabetes.
Several analytic approaches have been used to model the cost‐utility of novel insulins. Perhaps the most widely used is the IQVIA CORE Diabetes Model, a proprietary microsimulation consisting of multi‐chained Markov models for diabetes‐related complications. 20 , 21 While this approach provides a well‐validated 20 , 21 , 22 approximation of how input parameters influence the expected lifetime risk of diabetes‐related complications, it does not account for, or seek to assess, benefits relating to a reduction in treatment burden, nor the wider benefits that this may bring in terms of patient engagement. While HTA bodies will often highlight the importance of reducing the ‘treatment burden’ associated with a disease, they will seldom enumerate such benefits in a cost‐utility analysis.
2.1. Insulin therapy and drivers of value
The objective of insulin therapy is to achieve target levels of glycaemic control while minimising adverse complications such as hypoglycaemia. 23 Importantly, with appropriate dose titration, any modern insulin can enable patients to reach a pre‐specified level of glycaemic control. Where insulin regimens can differ is in secondary safety outcomes such as hypoglycaemia, weight gain, and injection site reactions. In clinical practice, these secondary outcomes are important drivers of regimen adherence and consequently impact the likelihood of achieving target levels of glycaemic control. Consequently, insulin clinical trials are required to adopt a treat‐to‐target design in which both intervention and control arms aim to achieve similar levels of glycaemic control, thereby allowing a comparison of these secondary effects. 24 This information provides clinicians with important insight into the tolerability of insulin regimens and, therefore, their potential utility within clinical practice. People with diabetes tend not to titrate their insulin dose to achieve an HbA1c target but to a level of hypoglycaemia they are prepared to tolerate, and the relationship between rates of hypoglycaemia and glycaemic control has been well characterised. 25 , 26 The treat‐to‐target trial design is not particularly informative for health economic assessment as the health benefit is restricted, by design, to a comparison of hypoglycaemia rates and other adverse side effects. Hypoglycaemia negatively impacts quality of life, increases fear and anxiety, and is associated with reduced productivity and increased healthcare costs. 27 However, the health economic value per hypoglycaemia event avoided is modest, with cost estimates ranging from £1.67 (non‐severe) to £2152 (severe) 28 and a per‐event disutility of 0.0033 (non‐severe) to 0.057 (severe). 29 , 30 While the burden of a severe event is more substantial, its incidence is relatively low (0.115 events per person per year 31 ). The true burden of hypoglycaemia becomes evident only when considering the compensatory behaviour (decreased insulin dose) that occurs from the desire to avoid hypoglycaemia. This represents a feedback system in which the positive benefits of good glycaemic control are undermined by the behavioural response associated with the patient's fear of hypoglycaemia. 27 Consequently, the features of insulin that drive health economic value in clinical practice are only partially reflected within the trial evidence, and any health technology assessment of an insulin is hamstrung by limitations arising from the mandated clinical trial design.
The impact of the treat‐to‐target clinical trial design on predicted cost‐effectiveness is easily demonstrated. SWITCH 1 and SWITCH 2 evaluated the efficacy and safety of insulin degludec within randomised, double‐blind, multi‐centre, two‐period crossover, treat‐to‐target clinical trials in patients with T1D and T2D, respectively. 32 , 33 In both trials, patients received either once‐daily degludec for 32 weeks followed by glargine U100 for a further 32 weeks, or vice versa. Both trials found that insulin degludec was associated with a significantly lower rate of overall symptomatic hypoglycaemia compared to insulin glargine U100 while achieving similar levels of glycaemic control.
Cost‐effectiveness analysis of the SWITCH trials utilised differing rates of hypoglycaemia and insulin dose requirements over a one‐year time horizon (the lack of any difference in glycaemic control rendering the need for long‐term projections unnecessary) have been reported. The incremental cost‐effectiveness ratios (ICERs) were £984 and £17 939 in T1D and T2D, respectively. 34 Both are below the £20 000 willingness‐to‐pay threshold used by NICE. The notable difference in results between T1D and T2D reflects the lower rates of hypoglycaemia in SWITCH 2. Post hoc analysis of the SWITCH trials compared how glycaemic control might be expected to vary between the two basal insulins for a given rate of hypoglycaemia and found that patients taking insulin degludec could achieve a mean HbA1c reduction of 0.70% (for T1D) and 0.96% (for T2D) before incurring an equivalent risk of hypoglycaemia to glargine U100. 35 As insulin dose titration strategies are designed to minimise the risk of hypoglycaemia, 36 this would imply that insulin degludec would likely deliver health economic benefits beyond hypoglycaemia alone in clinical practice.
Results from the Real‐World Clinical Treatment With Tresiba (ReFLeCT) study confirm this. 37 ReFLeCT was a multinational, multicentre, prospective, observational, single‐arm study in which patients with either T1D or T2D receiving insulin were switched to insulin degludec. The study demonstrated that the use of degludec was associated with significantly lower hypoglycaemia rates, better glycaemic control and significant improvements in treatment satisfaction. The cost‐effectiveness of switching to insulin degludec was either cost‐effective or dominant (cost‐saving with additional health benefit) versus other basal insulins for the treatment of T1D and T2D in Sweden and Italy. 38 , 39 Separately, in a single‐centre case series analysis in Wales, insulin degludec was prescribed for a series of consecutive patients experiencing treatment‐limiting problems with hypoglycaemia with either insulin detemir or insulin glargine. 34 Cited treatment challenges included nocturnal hypoglycaemia, recurrent hypoglycaemia, hypoglycaemia while driving, fear of hypoglycaemia, discomfort from injection access, variability in blood glucose levels and inflexibility in administration. Mean HbA1c reduction was 0.52% and 0.68% for T1D and T2D groups, respectively, mean insulin dose decreased in both T1D and T2D, and the mean rate of hypoglycaemic episodes per week decreased by over 90%. Cost‐effectiveness analysis undertaken for the T1D cohort using the CORE Diabetes Model indicated that insulin degludec was likely to be dominant. 40
In general, cost‐effectiveness analyses of ultra‐long‐acting insulins have typically demonstrated superior cost‐effectiveness in type 1 diabetes, compared with a basal/bolus regimen. 18 In type 2 diabetes, estimates of cost‐effectiveness differ by comparator (more cost‐effective when compared with basal/bolus regimens than basal/oral regimens) and are highly sensitive to input assumptions. 19 Adherence‐improving factors and equity are two relevant value elements associated with insulin that receive little consideration in cost‐effectiveness evaluations.
2.2. Diabetes technologies
Continuous glucose monitors (CGMs) are devices that measure the glucose levels in interstitial fluid continuously and send readings to a smart device in real time. Individuals can use this information to inform their food intake and insulin dosing to achieve a glucose reading within the target range.
Several studies have demonstrated that CGM increases time in range, improves glucose control, reduces the risk of hypoglycaemia and DKA and results in fewer hospital admissions for diabetes‐related complications. 41 , 42 , 43 It is important to note that this is entirely due to the behavioural‐modifying impact of the device. When combined with an insulin pump in an automated insulin delivery (AID) system, the time that individuals with T1D spend in the target range can resemble that of non‐diabetics. Individuals also see a significant reduction in glucose variability, hypoglycaemia, fear of hypoglycaemia, improved sleep, reduced anxiety and mental stress and ultimately improved quality of life. 44 , 45 These technologies also provide patients and healthcare professionals with information to guide personalised treatment strategies.
Evidence describing the health economics of contemporary diabetes management technologies generally finds them cost‐effective 46 despite considerable variability in reported cost‐effectiveness ratios. This variability is typically a function of applied assumptions, for example, the choice of HbA1c reduction selected, assuming equivalent hypoglycaemia rates between study groups and the use of data of limited generalisability. However, the timely use of insulin regimens, including new technological innovations such as once‐weekly basal insulins, the use of next‐generation smart insulins, insulin pumps, hybrid closed‐loop systems, and real‐time continuous glucose monitoring (rtCGM) has all been demonstrated to improve glucose control, patient‐reported outcomes, and patient‐important outcomes. 47 , 48 More recently, an oral nanotherapeutic formulation of insulin has demonstrated the potential to orally control blood glucose without hypoglycaemic episodes. 49 Consequently, the implementation of technology alongside insulin therapy can potentially address many of the barriers that exist regarding outcomes optimisation. Such a concept is caveated by the need to ensure equity of access while balancing affordability and clinical benefit.
2.3. The insulin value system
The burden of living with diabetes exerts significant psychosocial effects that impact self‐care, long‐term glycaemic control, risk of complications, and quality of life. 50 The negative emotional experience associated with the demands of diabetes is known as diabetes distress 51 and efforts to achieve optimal control can induce additional stress. Figure 2 seeks to visualise an insulin value system in which positive and negative feedback loops exist. The attainment of individualised glycaemic targets will deliver positive benefits to both patients, society, and the healthcare system, as the risk of diabetes‐related complications is reduced, thereby decreasing healthcare resource utilisation and improving quality‐adjusted life expectancy. Balancing this positive outcome is a negative feedback loop with intervention, provider, and patient‐related factors known to act as barriers to the timely initiation and/or intensification of insulin and contribute to poor adherence. 52 , 53
FIGURE 2.
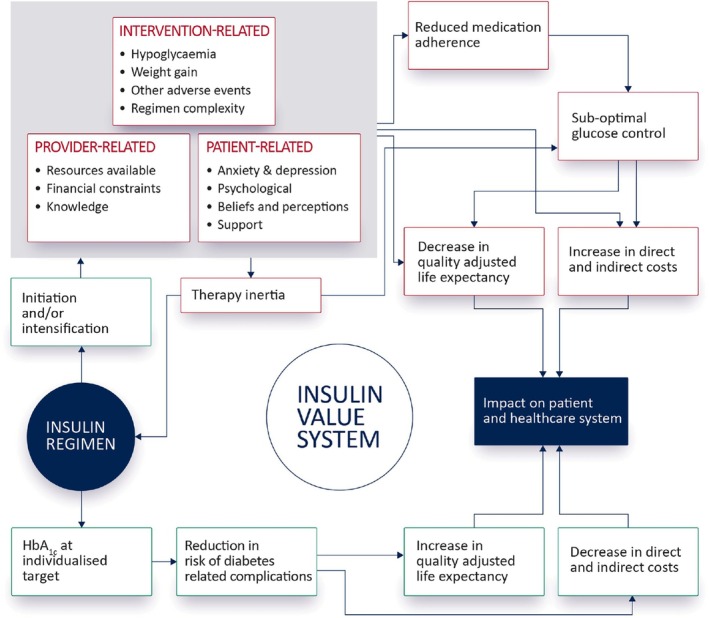
Conceptualising the interaction between the insulin regimen, the objectives of achieving target glycaemic control and the provider, patient and intervention‐related factors that can undermine the attainment of this objective.
Delays in insulin initiation occur due to concerns regarding hypoglycaemia, weight gain, fear of injections, the ongoing burden associated with the complexity of insulin regimens, and the presence of comorbid depression and anxiety. 52 Even after insulin initiation, the majority of patients do not achieve target glycaemic control. This has been attributed to, at least in part, titration inertia resulting from a lack of healthcare professional resources, including adequate assistance and education for patients, infrequent self‐measured blood glucose testing, and omission of insulin doses. Ongoing concerns regarding hypoglycaemia, weight gain, and the complexity of insulin regimens also contribute to delays in therapy intensification. 52
The implication of this system behaviour is that the expected health outcomes associated with an insulin regimen are reliant not only upon features of the insulin itself (intervention‐related) but also the psychosocial status of the patient and provider‐related resource constraints. Regimens that avoid hypoglycaemia and weight gain, minimise injection frequency, offer more flexibility and are easier to comply with are more likely to improve medication adherence and deliver value for both patients and the healthcare system.
The contribution of a formal CEA utilising an insulin treat‐to‐target trial design considered within a health technology assessment framework will consequently provide little insight into the true value of addressing factors that contribute to delays in timely insulin initiation, up‐titration, and intensification.
3. ADDRESSING THE RISING DEMANDS, COSTS, INEQUALITIES AND BARRIERS TO ACHIEVING OPTIMAL OUTCOMES
In 2021, the global number of people living with diabetes was estimated to be 529 million; the majority (96%) had T2D. Rising levels of obesity, poor nutrition, physical inactivity, ageing populations and longer life expectancy are driving the increase in expected diabetes prevalence, with 1.3 billion people projected to be living with diabetes in 2050. 54
The incidence of type 2 diabetes is strongly age‐related; consequently, as populations age, so will the burden of diabetes. Between 2025 and 2050, the number of people in the UK aged >60 years is projected to increase by 4.5 million, from 18 to 22.5 million. 55 Furthermore, the growing prevalence of childhood and adolescent obesity and diabetes incidence rates projected to surpass diabetes mortality rates provides insight into the expected 49% increase in diabetes prevalence in England by 2040. 56 Naturally, the economic implications are significant. A recent estimate of the direct annual cost associated with managing the 4.2 million people living with diabetes in the UK was £10.7 billion, approximately 60% related to the excess costs of complications with the remainder diagnosis and treatment‐related. 57 Indirect annual costs are also noteworthy, estimated at £3.3 billion, with absenteeism and early mortality resulting in 11.8 million sickness days and approximately 240 000 years of life lost, respectively.
There are neither quick fixes nor simple solutions to addressing the rising health demands of diabetes. Even the more focused question of addressing the rising demand for exogenous insulin therapy is not straightforward. It is, however, important to consider a more holistic approach to meeting this increasing demand for insulin therapy. For example, from a pathophysiological perspective, T2D represents a broad spectrum of disease with increasing recognition of the contribution of islet cell failure to the natural history of dysglycaemia, particularly pertinent in earlier onset T2D. The increased use of cardiorenal protective therapies such as sodium‐glucose co‐transporter 2 (SGLT2i) inhibitors, glucagon‐like receptor 1 receptor agonists (GLP1‐RAs) and nonsteroidal mineralocorticoid receptor antagonists (MRAs) are likely to translate into longer life expectancy with T2D. Consequently, the number of people requiring insulin therapy will rise.
The increasing uptake of these drugs, while potentially contributing to the future demands for insulin, may also have a role in offsetting some of the growing burden of insulin therapy. GLP1‐RAs are associated with clinically meaningful levels of weight loss 58 and improved insulin sensitivity, potentially resulting in a reduction in insulin dose requirement and regimen complexity. Furthermore, the use of GLP1‐RAs for the management of obesity has been shown to reduce the progression of dysglycaemia 59 and, in combination with coordinated diabetes prevention programmes, could contribute to a reduction in diabetes prevalence over time. 60 There is also an emerging focus on diabetes‐preventative strategies in susceptible individuals. The anti‐CD3 therapy teplizumab has been shown to delay the progression to symptomatic T1D in at‐risk individuals by up to 3 years 61 and pre‐clinical studies indicate antigen‐specific CAR T cells directed towards pancreatic β‐cells could prevent diabetes onset and progression. 62
The population‐level health challenge associated with diabetes appears overwhelmingly difficult. The tension between the affordability of disease management and the need for innovation to help drive improved outcomes and efficiency is difficult to reconcile. The length of time between disease onset and complications arising obscures the relationship between modifiable risk factors and disease outcomes. The complexity and logistical burden of diabetes management across primary, secondary and social care is not adequately reflected by considering cost alone. The only way to appreciate the value of optimised disease management is to simultaneously consider how all major components of diabetes and its management interact and evolve within healthcare as part of a dynamic system. Here we are specifically talking about the value system and, in particular, how a new technology will impact outcomes across the system not just those of relevance to an individual decision‐maker, such as the payer.
Consider the conventional approach to estimating the health economic value of delaying the onset of T1D. A cost‐effectiveness analysis of teplizumab conducted in the US captured benefits in terms of improvements in quality of life over the period of delay and the associated cost offsets: reduction in insulin requirement, consumables, clinic visits, and hypoglycaemia. 63 However, the implications of a diagnosis of T1D in children and young people (CYP) and their families are known to cause high rates of depression, stress, and adjustment problems. Furthermore, approximately 25% of those newly diagnosed with T1D in the UK present with diabetic ketoacidosis (DKA); this is associated with numerous deleterious outcomes, including cerebral oedema, neurocognitive deficits, shock, and arrhythmias. DKA at disease onset is associated with persistently elevated HbA1c and increased risk of future DKA, severe hypoglycaemia, 64 , 65 , 66 and a decline in IQ. Furthermore, the percentage of time blood glucose levels are outside the target range has also been shown to negatively impact brain development in adolescents with T1D. 67 Consequently, the value of a delay in T1D onset confers multiple benefits to numerous stakeholders over the short and longer term. Understanding the broad value of delay regarding service delivery capacity and coordination, education attainment, societal impact, long‐term costs, and outcomes requires a more holistic approach.
3.1. System‐wide assessment of value
Despite efforts to expand the definition of value from the payer perspective, assessing incremental value at the patient level offers little insight into how technologies might be utilised to address broader health and societal challenges. This implies that the application of public health economics may be more useful. 68 Certainly, there is no shortage of established diabetes models capable of providing insight into the impact of improved glycaemic control in T1D and T2D at the population level. 21 However, the problems facing healthcare systems involve a broader, interconnected, and dynamic set of circumstances; consequently, insight into practical and coordinated solutions can only be informed through a system‐wide assessment.
System dynamics is an established methodology well suited to supporting policy decisions involving complex and dynamic systems. 69 The approach combines system elements using mathematical models that capture relevant interactions and feedback loops. The purpose is to understand how the system responds to changes in model inputs and assumptions. System dynamics has been extensively deployed in healthcare to improve the understanding of disease management strategies, delivery and workforce planning, patient flows, and disaster planning. 70 , 71 , 72 , 73 The use of systems approaches within public health economic modelling has also been advocated for considering the social determinants of health, incorporating models of behaviour, social interactions and equity. 74
In diabetes, the Center for Disease Control (CDC) has used system dynamics to provide insight into the key characteristics of the US diabetes population 75 identifying the significance of obesity in driving the growth of diabetes, how improved disease management alone is insufficient to reduce diabetes prevalence over the longer term and the substantial delay expected between primary prevention initiatives and realising any benefit in diabetes outcomes. The impact of new treatments and predictive technologies on T2D prevalence and healthcare expenditure has also been assessed, providing insights into the benefit of improved diabetes control and the paradoxical increase in T2D prevalence and overall cost that arises due to patients living longer. 76 System dynamics has been used to identify critical success factors when developing a community diabetes care programme 77 and assessing the role of economic, social, and environmental factors on chronic disease prevalence. 78
Figure 3 conceptualises a diabetes‐specific healthcare system where the interaction between population dynamics, disease management and access to healthcare delivery is considered alongside the patient, society and environmental factors.
FIGURE 3.
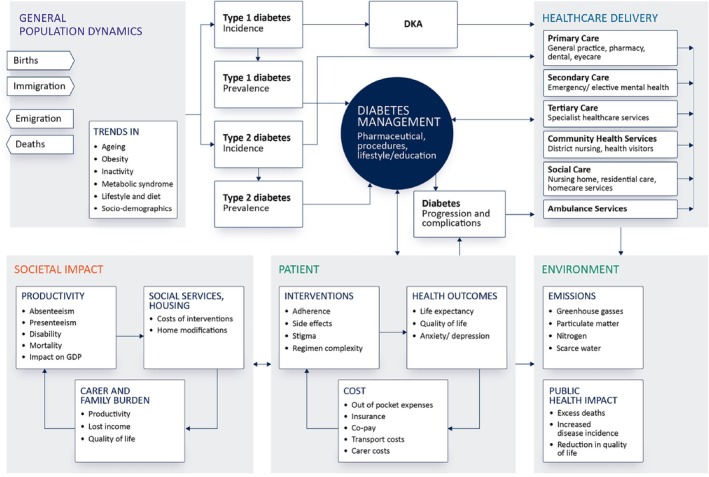
Conceptualising the healthcare system with respect to diabetes management in which the interaction between population dynamics, disease management and access to healthcare delivery is considered alongside the patient, society and environmental factors.
Supplementing this system map are a set of interconnected features of the system that can drive outcomes of value considered relevant to the various stakeholders within healthcare; for example:
Population: Birth rates, death rates, migration, disease incidence (including relevant co‐morbidities), prevalence, duration of disease, socio‐demographic and co‐morbidity indices.
Healthcare delivery: Number of primary care visits (physician, nurse, dietician, etc.), number of inpatient/outpatient episodes, length of hospital stay, transport costs, proportion of medical patients using surgical beds, number of premature hospital discharges, requirement to purchase private social care capacity, waiting lists, readmission rates, total costs, total demand.
Diabetes management: The use of insulin itself poses specific system‐wide challenges. The global supply of insulin relies on a limited number of manufacturers, increasing the risk of supply shortages associated with manufacturing issues and geopolitical tensions. Insulin is a cold chain product whereby temperature excursions can render the insulin ineffective. Consequently, adequate infrastructure, robust transportation and distribution channels, the availability of cold storage and the strict adherence to cold storage protocols are all essential. The fragility of the insulin supply chain has the potential to directly impact blood glucose control, the incidence of DKA, and increase fear and anxiety associated with running out of insulin. Supply shortages can also drive insulin price inflation, placing further strains on both individuals and healthcare systems. 79
Patient: Length of life lived, quality of life, impact on family/carers, inability to work, impact on pension, accessing care, medical care costs, transport costs, out‐of‐pocket expenditure, income lost, ability to live independently, impact on educational achievement, requirement for future healthcare interventions.
Societal impact: Absenteeism, presenteeism, macroeconomic impact, social care capacity.
Environmental: Greenhouse gas emissions, particulate matter, water usage.
Considering a systems approach to informing value decisions has a number of advantages. Firstly, it facilitates the linkage of metrics relevant to individual stakeholders. A payer will consider the cost saving from avoiding a hospital admission as relevant, while for a provider of care, the corresponding reduction in demand it confers is more meaningful. Secondly, it can illustrate how decisions made in one part of the system will likely impact other parts; for example, the requirement to balance the pharmacy budget (prioritising cheaper insulins) may have unintended consequences elsewhere in terms of disease management, such as patient adherence, additional hospital admissions, quality of life, and lost productivity. Thirdly, it can provide insight into the system‐wide burden of clinical events that occur relatively infrequently. For example, the per‐patient risk of hospitalisation for DKA is low, in the region of 7–10 events per 100 000 person‐years 80 ; however, at the population level, its burden is high. An ecological study of diabetes‐related hospital admissions in England and Wales from 1999 to 2020 reported a total of 1 757 892 hospital admissions, with 47.1% of these admissions attributed to T1D. 81 Approximately half (47.6%) of all T1D hospital admissions were DKA‐related (the equivalent statistics for T2D were 11.3%). Similarly, the rate of severe hypoglycaemia is relatively low, in the region of 12 and 2 per 1000 patient years for T1D and T2D, respectively 82 ; however, the burden in absolute terms is large, with 101 475 hospital admissions for hypoglycaemia in England between 2005 and 2014. 83 Understanding the contribution of innovation to improving healthcare outcomes is also impacted by the exclusion of unrelated costs in technology assessments. 84 , 85 Life‐extending treatments will ultimately result in additional medical costs throughout additional life. It is argued that where these additional costs occur due to unrelated diseases, their inclusion would negatively impact the cost‐effectiveness of life‐extending treatments; however, in diabetes, this has been disputed. 86 Such unrelated costs reflect real resource utilisation that will ultimately be borne by the healthcare system, and while arguments for and against their inclusion in technology assessments continue, the need to consider population and disease dynamics in totality is imperative for system‐wide capacity planning.
The information necessary to develop a quantitative tool suitable for assessing disease dynamics at the population level is typically available. For example, traditional cost‐effectiveness models provide insight into the natural progression of a disease. Supplementing this with population parameters (births, deaths, net migration, age structure of the population), and finally disease incidence and prevalence data is sufficient to construct such a tool. While a system‐wide model could be of use in describing disease burden and unmet need for HTA, its use is more closely aligned to supporting broader guideline and policy development.
3.2. Addressing rising demands
Addressing the rising demands that diabetes will impose upon the healthcare system will require the simultaneous execution of multiple strategies that acknowledge population dynamics, healthcare delivery constraints, the role of innovation, and funding requirements. In England, for example, the number of people living with major illnesses is expected to increase by a third to 2.5 million by 2040, representing 1 in 5 (9.1 million) of the adult population. 87 Most will be aged 70 years and older, and people will likely live for longer with major illness (11.2 years in 2019 to 12.6 years in 2040). The competition for healthcare resources will consequently increase as the number living with major illnesses also increases (by over 30%); meanwhile, the number of tax‐paying people of working age is expected to grow by just 4%. Therefore, strategies aimed at primary prevention are crucial. The prevalence of obesity continues to increase in many populations and is an important risk factor for T2D, cardiovascular disease, musculoskeletal disorders, and certain cancers. Newer pharmacotherapies for obesity treatment, such as high‐dose semaglutide and tirzepatide, have demonstrated real‐world effectiveness close to the efficacy reported in their respective clinical trials 88 , 89 , 90 ; yet they remain under‐prescribed. 91 The complexity of obesity science, the education and management of patients, and concerns regarding affordability are likely to act as barriers to uptake. Primary prevention of T2D is also possible in at‐risk populations, and diabetes prevention programs have demonstrated effectiveness in real‐world scenarios. 60 While the financial cost to the healthcare system associated with primary prevention would be high, the opportunity cost is likely greater.
Optimising disease management requires multidisciplinary teams, comprising clinicians, nurses, dieticians, pharmacists, and diabetes education and care coordinators. Accounting for patient‐specific characteristics to develop individualised plans and utilising technologies that address relevant barriers to care: fear of hypoglycaemia, fear of needles, weight gain, regimen complexity, and anxiety will require a whole system perspective on healthcare value and an appreciation of the interconnectivity of stakeholder needs within healthcare.
Patient circumstances also influence outcomes. Socioeconomic deprivation, ethnicity, mental health conditions, and physical and learning disabilities are known to drive inequality in healthcare. 92 This impacts the risk of developing diabetes, access to structured education and care, access to new technology, glycaemic control, and risk of hypoglycaemia and DKA, ultimately increasing both morbidity and mortality. Coordinated government action to address inequalities in both society and healthcare is therefore required. In 2010, the Marmot report 93 advocated evidence‐based strategies to address inequalities in health and society by focusing expenditure on early years development, training and support for young people, a minimum income for healthy living, a tax system to strengthen incentives to work, and plans to reduce social isolation. National performance indicators such as life expectancy and healthy life expectancy were also proposed. Since then, health inequalities have actually worsened, disproportionately affecting the most deprived 94 and in the absence of coordinated government action, diabetes healthcare professionals have been called upon to develop culturally and individually appropriate structured education, encourage hard‐to‐reach groups to engage with the healthcare system, ensure patients from all backgrounds can access the technologies they need, and provide carers of those with intellectual disabilities the training and guidance they require. 92
One can argue, therefore, that the solutions to addressing inequalities and optimising disease outcomes in diabetes have been identified. What is lacking is a system‐wide appreciation of the economic and humanistic benefits associated with coordinated action and the consequences of inaction. The assessment of value at the system level provides a mechanism for understanding where investment in health will deliver a return. We need to simultaneously consider value at the patient, population, regional, and national levels. For example, the therapeutic effect associated with utilising a novel insulin or device should deliver similar clinical outcomes across patients, but the benefit would be different in younger versus older patients. In the former, a benefit might be realised in terms of educational attainment, future earnings, improved quality of life, and reduced healthcare expenditure in the longer term. In the latter, benefit may be driven by reduced carer burden and improved quality of life in the short term. Furthermore, the value of these benefits is conditional upon the state of the system at any given time and the pressures/constraints that exist locally and nationally. Therefore, value has to consider the availability of resources and an understanding of the requirements to effectively deliver healthcare accommodating local and national population dynamics. Recognising there is a financial, societal, and economic cost associated with maintaining the status quo, we first need to quantify this cost before we can fully appreciate the value of intervening. Key to meeting rising demands, costs, and inequalities in the more immediate term is the need to support and ensure optimal insulin treatment initiation, intensification, and adherence. This requires aligning the insulin regimen to the patient's concerns and unique circumstances. Importantly, convenience and treatment satisfaction are often not considered valuable features of insulin therapy; not only do they have value, but they are also essential to addressing rising demands.
CONFLICT OF INTEREST STATEMENT
Phil McEwan is an employee of Health Economics and Outcomes Research Ltd., that has provided research consultancy for AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Takeda and Sanofi. Marc Evans has previously received consulting fees or honoraria from AstraZeneca, Novo Nordisk, Takeda, Napp, MSD, Sunovion and Novartis.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/dom.16488.
ACKNOWLEDGEMENTS
This article was commissioned by the Editor as part of a Themed issue on Biosimilar insulins made possible by funding from Gan & Lee Inc. Sponsor identity was not disclosed to authors prior to publication. PM and ME received no funding for this manuscript. PM and ME conceptualised, wrote and reviewed the manuscript and provided approval of the final manuscript for publication.
McEwan P, Evans M. The health economics of insulin therapy: How do we address the rising demands, costs, inequalities and barriers to achieving optimal outcomes. Diabetes Obes Metab. 2025;27(Suppl. 5):24‐35. doi: 10.1111/dom.16488
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. World Health Organization . Global spending on health 2020: weathering the storm. 2020.
- 2. Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. [DOI] [PubMed] [Google Scholar]
- 3. Drummond M, Stoddart G, Labelle R, Cushman R. Health economics: an introduction for clinicians. Ann Intern Med. 1987;107(1):88–92. [DOI] [PubMed] [Google Scholar]
- 4. Neumann PJ, Garrison LP, Willke RJ. The history and future of the “ispor value flower”: Addressing limitations of conventional cost‐effectiveness analysis. Value Health. 2022;25(4):558–565. [DOI] [PubMed] [Google Scholar]
- 5. Simon HA, Egidi M, Marris R. Economics, Bounded Rationality and the Cognitive Revolution. E. Elgar; 1995. [Google Scholar]
- 6. Diabetes UK . Early diagnosis of children with type 1 diabetes. 2015.
- 7. Cahn A, Miccoli R, Dardano A, Del Prato S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3(8):638–652. [DOI] [PubMed] [Google Scholar]
- 8. Farmer RE, Beard I, Raza SI, et al. Prescribing in type 2 diabetes patients with and without cardiovascular disease history: a descriptive analysis in the UK cprd. Clin Ther. 2021;43(2):320–335. [DOI] [PubMed] [Google Scholar]
- 9. Beran D, Lazoporras M, Mba CM, Mbanya JC. A global perspective on the issue of access to insulin. Diabetologia. 2021;64:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krause M, De Vito G. Type 1 and type 2 diabetes mellitus: commonalities, differences and the importance of exercise and nutrition. 2023. [DOI] [PMC free article] [PubMed]
- 11. Basu S, Yudkin JS, Kehlenbrink S, et al. Estimation of global insulin use for type 2 diabetes, 2018–30: a microsimulation analysis. Lancet Diabetes Endocrinol. 2019;7(1):25–33. [DOI] [PubMed] [Google Scholar]
- 12. Wilson LM, Castle JR. Recent advances in insulin therapy. Diabetes Technol Ther. 2020;22(12):929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bajaj HS, Goldenberg RM. Insulin icodec weekly: a basal insulin analogue for type 2 diabetes. touchREV Endocrinol. 2023;19(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayes AJ, Jose Leal AM, Gray RRH, Clarke PM. Ukpds outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom prospective diabetes study: Ukpds 82. Diabetologia. 2013;56:1925–1933. [DOI] [PubMed] [Google Scholar]
- 15. Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and validation of risk equations for complications of type 2 diabetes (recode) using individual participant data from randomised trials. Lancet Diabetes Endocrinol. 2017;5(10):788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davies MJ, Drexel H, Jornayvaz FR, Pataky Z, Seferovi'c PM, Wanner C. Cardiovascular outcomes trials: a paradigm shift in the current management of type 2 diabetes. Cardiovasc Diabetol. 2022;21(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McEwan P, Morgan AR, Boyce R, et al. The costeffectiveness of dapagliflozin in treating high‐risk patients with type 2 diabetes mellitus: an economic evaluation using data from the declaretimi 58 trial. Diabetes Obes Metab. 2021;23(4):1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saunders H, Loong D, Mishra S, et al. The cost‐effectiveness of intermediate‐acting, long‐acting, ultralong‐acting, and biosimilar insulins for type 1 diabetes mellitus: a systematic review. Value Health. 2022;25(7):1235–1252. [DOI] [PubMed] [Google Scholar]
- 19. Shafie AA, Ng CH, Tan YP, Chaiyakunapruk N. Systematic review of the cost effectiveness of insulin analogues in type 1 and type 2 diabetes mellitus. Pharmacoeconomics. 2017;35:141–162. [DOI] [PubMed] [Google Scholar]
- 20. Palmer AJ, Mount Hood 5 Modeling Group , Clarke P, et al. Computer modeling of diabetes and its complications: a report on the fifth mount hood challenge meeting. Value Health. 2013;16(4):670–685. [DOI] [PubMed] [Google Scholar]
- 21. Palmer AJ, Si L, Tew M, et al. Computer modeling of diabetes and its transparency: a report on the eighth mount hood challenge. Value Health. 2018;21(6):724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the ims core diabetes model. Value Health. 2014;17(6):714–724. [DOI] [PubMed] [Google Scholar]
- 23. 6. Glycemic goals and hypoglycemia: standards of care in diabetes—2025. Diabetes Care. 2025;48(Supplement 1):S128–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garber AJ. Treat‐to‐target trials: uses, interpretation and review of concepts. Diabetes Obes Metab. 2014;16(3):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Home PD. The vexed question of hypoglycaemia and health economics. Diabetes Obes Metab. 2014;16(4):289–293. [DOI] [PubMed] [Google Scholar]
- 26. Mullins P, Sharplin P, Ykijarvinen H, Riddle MC, Haring H. Negative binomial meta‐regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven phase iii and iv studies of insulin glargine compared with neutral protamine hagedorn insulin in type 1 and type 2 diabetes mellitus. Clin Ther. 2007;29(8):1607–1619. [DOI] [PubMed] [Google Scholar]
- 27. Fidler C, Christensen TE, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality‐of‐life, and impact on costs. J Med Econ. 2011;14(5):646–655. [DOI] [PubMed] [Google Scholar]
- 28. Parekh WA, Ashley D, Chubb B, Gillies H, Evans M. Approach to assessing the economic impact of insulin‐related hypoglycaemia using the novel local impact of hypoglycaemia tool. Diabet Med. 2015;32(9):1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health‐related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534. [DOI] [PubMed] [Google Scholar]
- 30. Xie X, Guo J, Bremner KE, Wang M, Shah BR, Volodin A. Review and estimation of disutility for joint health states of severe and nonsevere hypoglycemic events in diabetes. J Comparat Effect Ness Res. 2021;10(13):961–974. [DOI] [PubMed] [Google Scholar]
- 31. Wang H, Donnan PT, Leese CJ, et al. Temporal changes in frequency of severe hypoglycemia treated by emergency medical services in types 1 and 2 diabetes: a population‐based data‐linkage cohort study. Clin Diabetes Endocrinol. 2017;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lane W, Bailey TS, Gerety G, et al. Group Information, and SWITCH 1. Effect of insulin degludec vs insulin glargine u100 on hypoglycemia in patients with type 1 diabetes: the switch 1 randomized clinical trial. JAMA. 2017;318(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wysham C, Bhargava A, Chaykin L, et al. Effect of insulin degludec vs insulin glargine u100 on hypoglycemia in patients with type 2 diabetes: the switch 2 randomized clinical trial. JAMA. 2017;318(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evans M, Mehta R, Gundgaard J, Chubb B. Cost‐effectiveness of insulin degludec vs. insulin glargine u100 in type 1 and type 2 diabetes mellitus in a UK setting. Diabetes Ther. 2018;9(5):1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Philistsimikas A, Lane W, Pedersenbjergaard U, et al. The relationship between hba1c and hypoglycaemia in patients with diabetes treated with insulin degludec versus insulin glargine 100. Diabetes Obes Metab. 2020;22(5):779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuritzky L, Reid TS, Wysham CH. Practical guidance on effective basal insulin titration for primary care providers. Clin Diabetes. 2019;37(4):368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fadini GP, Feher M, Hansen TK, et al. Switching to degludec from other basal insulins is associated with reduced hypoglycemia rates: a prospective study. J Clin Endocrinol Metab. 2019;104(12):5977–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jendle J, Ericsson A, Ekman B, et al. Real‐world cost‐effectiveness of insulin degludec in type 1 and type 2 diabetes mellitus from a swedish 1‐year and long‐term perspective. J Med Econ. 2020;23(11):1311–1320. [DOI] [PubMed] [Google Scholar]
- 39. Haldrup S, Lapolla A, Gundgaard J, Wolden ML. Cost‐effectiveness of switching to insulin degludec from other basal insulins in real‐world clinical practice in Italy. J Med Econ. 2020;23(3):271–279. [DOI] [PubMed] [Google Scholar]
- 40. Evans M, McEwan P. Clinical and cost‐effectiveness of insulin degludec: from clinical trials to clinical practice. J Comp Eff Res. 2015;4(3):279–286. [DOI] [PubMed] [Google Scholar]
- 41. Lind N, Christensen MB, Hansen DL, Nørgaard K. Comparing continuous glucose monitoring and blood glucose monitoring in adults with inadequately controlled, insulin‐treated type 2 diabetes (steno2tech study): a 12‐month, single‐center, randomized controlled trial. Diabetes Care. 2024;47(5):881–889. [DOI] [PubMed] [Google Scholar]
- 42. Nathanson D, Eeg‐Olofsson K, Spelman T, et al. Intermittently scanned continuous glucose monitoring compared with blood glucose monitoring is associated with lower hba1c and a reduced risk of hospitalisation for diabetes‐related complications in adults with type 2 diabetes on insulin therapies. Diabetologia. 2025;68(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karges B, Tittel SR, Bey A, et al. Continuous glucose monitoring versus blood glucose monitoring for risk of severe hypoglycaemia and diabetic ketoacidosis in children, adolescents, and young adults with type 1 diabetes: a population‐based study. Lancet Diabetes Endocrinol. 2023;11(5):314–323. [DOI] [PubMed] [Google Scholar]
- 44. Ng SM, Wright NP, Yardley D, et al. Real world use of hybrid‐closed loop in children and young people with type 1 diabetes mellitus—a national health service pilot initiative in England. Diabet Med. 2023;40(2):e15015. [DOI] [PubMed] [Google Scholar]
- 45. Crabtree TSJ, Griffin TP, Yap YW, et al. Hybrid closed‐loop therapy in adults with type 1 diabetes and above target hba1c: a real‐world observational study. Diabetes Care. 2023;46(10):1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pease A, Zomer E, Liew D, Lo C, Earnest A, Zoungas S. Cost‐effectiveness of health technologies in adults with type 1 diabetes: a systematic review and narrative synthesis. Syst Rev. 2020;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boughton CK, Hartnell S, Lakshman R, et al. Fully closed‐loop glucose control compared with insulin pump therapy with continuous glucose monitoring in adults with type 1 diabetes and suboptimal glycemic control: a single‐center, randomized, crossover study. Diabetes Care. 2023;46(11):1916–1922. [DOI] [PubMed] [Google Scholar]
- 48. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–374. [DOI] [PubMed] [Google Scholar]
- 49. Hunt NJ, Lockwood GP, Heffernan SJ, et al. Oral nanotherapeutic formulation of insulin with reduced episodes of hypoglycaemia. Nat Nanotechnol. 2024;19(4):534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ő ry F, Kiss B L'a'o, Zsid'o A'a N, Teleki SÁ. Conquering diabetes by overcoming psychological barriers and embracing health. Sci Rep. 2024;14(1):32104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes related distress. Diabetes Care. 1995;18(6):754–760. [DOI] [PubMed] [Google Scholar]
- 52. Russell‐Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20(3):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Evans M, Morgan AR, Bain SC. One hundred years of insulin: value beyond price in type 2 diabetes mellitus. Diabetes Ther. 2021;12(6):1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. 55GBD 2021 Diabetes collaborators . Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet. 2023;402(10397):203–234. doi: 10.1016/S0140-6736(23)01301-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. The Centre Ageing for Better . The State of Ageing in 2023. The Centre for Ageing Better; 2023. [Google Scholar]
- 56. Rachet‐Jacquet L, Head A, Kypridemos C, et al. Health in 2040: Projected Patterns of Illness in England. Technical Report. The Health Foundation; 2023. [Google Scholar]
- 57. Hex N, MacDonald R, Pocock J, et al. Estimation of the direct health and indirect societal costs of diabetes in the UK using a cost of illness model. Diabet Med. 2024;41(9):e15326. [DOI] [PubMed] [Google Scholar]
- 58. Li H, Guanzheng Y, Huang Q, et al. Efficacy and safety of glp‐1ras for people with obesity: a systematic review based on rct and bayesian network meta‐analysis. Biomed Pharmacother. 2024;171:116150. [DOI] [PubMed] [Google Scholar]
- 59. Kahn SE, Deanfield JE, Jeppesen OK, et al. Effect of semaglutide on regression and progression of glycemia in people with overweight or obesity but without diabetes in the select trial. Diabetes Care. 2024;47(8):1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bahari NI, Ahmad N, Mahmud MH, et al. Issues and challenges in the primary prevention of type 2 diabetes mellitus: a systematic review. J Prev Dent. 2023;44(1):105–125. [DOI] [PubMed] [Google Scholar]
- 61. Ramos EL, Dayan CM, Chatenoud L, et al. Teplizumab and β‐cell function in newly diagnosed type 1 diabetes. N Engl J Med. 2023;389(23):2151–2161. [DOI] [PubMed] [Google Scholar]
- 62. Mohammadi V, Maleki AJ, Nazari M, et al. Chimeric antigen receptor (car)‐based cell therapy for type 1 diabetes mellitus (t1dm); current progress and future approaches. Stem Cell Rev Rep. 2024;20(3):585–600. [DOI] [PubMed] [Google Scholar]
- 63. Mital S, Nguyen HV. Cost effectiveness of teplizumab for prevention of type 1 diabetes among different target patient groups. Pharmacoeconomics. 2020;38(12):1359–1372. [DOI] [PubMed] [Google Scholar]
- 64. Karges B, Prinz N, Placzek K, et al. A comparison of familial and sporadic type 1 diabetes among young patients. Diabetes Care. 2021;44(5):1116–1124. [DOI] [PubMed] [Google Scholar]
- 65. Smith LB, Liu X, Johnson SB, et al. Family adjustment to diabetes diagnosis in children: can participation in a study on type 1 diabetes genetic risk be helpful? Pediatr Diabetes. 2018;19(5):1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long‐term glycemic control. Diabetes Care. 2017;40(9):1249–1255. [DOI] [PubMed] [Google Scholar]
- 67. Besser REJ, Ng SM, Gregory JW, Dayan CM, Randell T, Barrett T. General population screening for childhood type 1 diabetes: is it time for a UK strategy? Arch Dis Child. 2022;107(9):790–795. [DOI] [PubMed] [Google Scholar]
- 68. Edwards RT, Charles JM, Lloyd‐Williams H. Public health economics: a systematic review of guidance for the economic evaluation of public health interventions and discussion of key methodological issues. BMC Public Health. 2013;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bala BK, Arshad FM, Noh KM, et al. System dynamics. Modell Simul. 2017;274:15–34. [Google Scholar]
- 70. Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health. 2006;96(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Morshed AB, Kasman M, Heuberger B, Hammond RA, Hovmand PS. A systematic review of system dynamics and agent‐based obesity models: evaluating obesity as part of the global syndemic. Obes Rev. 2019;20(Suppl 2):161–178. [DOI] [PubMed] [Google Scholar]
- 72. Lee JT, Crettenden I, Tran M, et al. Methods for health workforce projection model: systematic review and recommended good practice reporting guideline. Hum Resour Health. 2024;22(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Russell E, Johnson B, Heidi Larsen M, et al. Health systems in context: a systematic review of the integration of the social determinants of health within health systems frameworks. Rev Panam Salud Publica. 2013;34(6):461–467. [PubMed] [Google Scholar]
- 74. Squires H, Chilcott J, Akehurst R, Burr J, Kelly MP. A systematic literature review of the key challenges for developing the structure of public health economic models. Int J Public Health. 2016;61(3):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jones AP, Homer JB, Murphy DL, Essien JDK, Milstein B, Seville DA. Understanding diabetes population dynamics through simulation modeling and experimentation. Am J Public Health. 2006;96(3):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hirani A, Guergachi A, Keshavjee K. System dynamics modeling for diabetes treatment and prevention planning. Stud Health Technol Inform. 2025;322:83–84. [DOI] [PubMed] [Google Scholar]
- 77. Homer J, Hirsch G, Minniti M, Pierson M. Models for collaboration: how system dynamics helped a community organize cost‐effective care for chronic illness. Syst Dyn Rev. 2004;20(3):199–222. [Google Scholar]
- 78. Brittin J, Araz OM, Nam Y, Huang TT‐K. A system dynamics model to simulate sustainable interventions on chronic disease outcomes in an urban community. J Simul. 2015;9(2):140–155. [Google Scholar]
- 79. Haji MHAA. Enhancing insulin supply chain resilience: a critical importance for diabetes management. Global J Obes Diabetes Metab Syndr. 2023;10(1):9–13. [Google Scholar]
- 80. Ebrahimi F, Kutz A, Christ ER, Szinnai G. Lifetime risk and health‐care burden of diabetic ketoacidosis: a population‐based study. Front Endocrinol (Lausanne). 2022;13:940990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. AbuHammad GAR, Naser AY, Hassouneh LKM. Diabetes mellitus‐related hospital admissions and prescriptions of antidiabetic agents in England and Wales: an ecological study. BMC Endocr Disord. 2023;23(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Berthon W, McGurnaghan SJ, Blackbourn LAK, et al. Ongoing burden and recent trends in severe hospitalised hypoglycaemia events in people with type 1 and type 2 diabetes in Scotland: a nation‐wide cohort study 2016–2022. Diabetes Res Clin Pract. 2024;210:111642. [DOI] [PubMed] [Google Scholar]
- 83. Zaccardi F, Davies MJ, Dhalwani NN, et al. Trends in hospital admissions for hypoglycaemia in England: a retrospective, observational study. Lancet Diabetes Endocrinol. 2016;4(8):677–685. [DOI] [PubMed] [Google Scholar]
- 84. Morton A, Adler AI, Bell D, et al. Unrelated future costs and unrelated future benefits: reflections on nice guide to the methods of technology appraisal. Health Econ. 2016;25(8):933–938. [DOI] [PubMed] [Google Scholar]
- 85. Perry‐Duxbury M, Lomas J, Asaria M, van Baal P. The relevance of including future healthcare costs in cost‐effectiveness threshold calculations for the UK NHS. Pharmacoeconomics. 2022;40(2):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhao T, Tew M, Feenstra T, et al. The impact of unrelated future medical costs on economic evaluation outcomes for different models of diabetes. Appl Health Econ Health Policy. 2024;22(6):861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Watt T, Raymond A, Rachet‐Jacquet L, et al. Health in 2040: Projected Patterns of Illness in England. Health Foundation; 2023. [Google Scholar]
- 88. Kelly MS, Scopelliti EM, Goodson KE, Lo CMA, Nguyen HX, Simon B. Real‐world evaluation of the effects of tirzepatide in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2024;26(12):5661–5668. [DOI] [PubMed] [Google Scholar]
- 89. Tzoulis P, Batavanis M, Baldeweg S. A real‐world study of the effectiveness and safety of semaglutide for weight loss. Cureus. 2024;16(5):e59558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Marassi M, Fadini GP. Real‐world evidence on oral semaglutide for the management of type 2 diabetes. A narrative review for clinical practice. Clin Ther. 2025;47(1):102–110. [DOI] [PubMed] [Google Scholar]
- 91. Khidhir A, Aladesua T. Underprescribing of cardioprotective novel antidiabetic drugs in marginalized patients with type ii diabetes mellitus. Eur Heart J. 2023;44(Supplement 2):ehad655–3006. [Google Scholar]
- 92. Kilvert A, Fox C. Health inequalities and diabetes. Pract Diabetes. 2023;40(1):19–24a. [Google Scholar]
- 93. Marmot M. Fair Society, Healthy Lives. Oxford University Press; 2013. [Google Scholar]
- 94. Marmot M. Health equity in England: the marmot review 10 years on. BMJ. 2020;368:m693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


