Abstract
We report a straightforward synthesis of N-unsubstituted five-, six-, and seven-membered cyclic carbamates from amino alcohols and CO2, employing user-friendly propanephosphonic acid anhydride (T3P) as a dehydrating agent. Demanding substrates, such as amino alcohols bearing tertiary hydroxyl groups, cyclize in good yields under mild conditions, providing access to fused and spirocyclic carbamates. Mechanistic studies indicate that the amino alcohols react with CO2, forming poorly soluble salts, which are then solubilized by catalytic superbase 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU).
Nitrogen-containing heterocycles are a key structural motif in many pharmaceuticals and agrochemicals. − Cyclic carbamates, a subgroup of N-heterocycles, have been widely utilized as peptide bond surrogates due to their pharmacokinetic improving properties and good metabolic stability. , Indeed, cyclic carbamates of varying ring-sizes are present in several important pharmaceuticals (Scheme A). −
1. Applications and Synthesis of N-Unsubstituted Cyclic Carbamates.

Cyclic carbamates bearing an unsubstituted nitrogen atom may be the desired end-product, but this nitrogen atom also provides a synthetic handle in the development of pharmaceutical libraries, a strategy realized in the development of anacetrapib and GSK279874 (Scheme B). , In this context, the easily accessed and versatile amino alcohols are an excellent class of starting materials. ,
To access the above-mentioned privileged structures from amino alcohols, we identified several important criteria that the ideal cyclization reaction should fulfill (Scheme C). The method should be able to produce N-unsubstituted cyclic carbamates (i) and offer flexibility in ring-size (ii). We had previously found difficulty in cyclizing amino alcohols bearing tertiary alcohols, which would be important to access spirocyclic structures such as GSK279874 (iii). Similarly, we wanted less nucleophilic anilines to be compatible to obtain fused structures such as Efavirenz (iv). Finally, considering that GSK279874 and Efavirenz bear chiral centers at the alcohol, we wanted cyclization to proceed in a stereoselective manner (v).
The state-of-the-art route to cyclic carbamates employs carbonyl diimidazole (CDI) as a carbonyl source (Scheme D). While this method readily provides five-membered rings, i.e., 2-oxazolidinones, diminished yields are observed for the corresponding six-membered cyclic carbamates due to competing urea formation. This is even more pronounced for seven-membered cyclic carbamates, which are only obtained in trace amounts.
Considering alternative carbonyl sources, we identified CO2 as a safe, abundant, and readily available carbonyl source, which would give it a competitive edge over conventional phosgene-based reagents. − Synthetic methods yielding 2-oxazolidinones from CO2 are well established. , In contrast, there are significantly fewer examples of CO2-based methods to access 6-membered cyclic carbamates. − These methods require engineered starting materials, particularly for N-unsubstituted products. − Examples of seven-membered carbamates from CO2 are rare. , To the best of our knowledge, there exists no CO2-based method to N-unsubstituted 7-membered cyclic carbamates. This bias in chemical space likely stems from the fact that N-substitution significantly accelerates cyclization, whereas increasing ring-size beyond five will have the opposite effect. , In other words, formation of N-unsubstituted six- or seven-membered cyclic carbamates is challenging.
Seeking to overcome the challenges listed above, we were attracted to a study where amino alcohols were cyclized in the presence of CO2 and a dehydrating agent under cryogenic conditions (Scheme E). While diastereomeric mixtures were obtained, the transformation showed good stereoselectivity when using phosphorus-based reagents, particularly diphenylphosphoryl azide (DPPA).
However, the carbamoyl azide intermediate did not always cyclize (Scheme F). This lead us to consider highly reactive isocyanates, which were generated from amino alcohols and CO2 under dehydrative Mitsunobu conditions, using tributylphosphine, an air-sensitive, toxic (LD50 750 mg kg–1), and pyrophoric liquid with a nauseating odor. The reaction scope centered on 2-oxazolidinones, with only one example of a six-membered cyclic carbamate. Nevertheless, we saw the potential of an isocyanate-based strategy in driving challenging cyclizations.
Herein we report synthesis of valuable N-unsubstituted 2-oxazolidinones and elusive six- and seven-membered cyclic carbamates using a nontoxic dehydrating agent (Scheme G).
We began our study by selecting T3P as the dehydrating agent due to its low toxicity (LD50 > 2000 mg kg–1) and an established use on industrial scale (Table , graphic). The oxidized form of T3P (T3PO–2) is highly water-soluble and is therefore easily removed, in contrast to conventionally used phosphine oxides. We noted that T3P was recently used in a CO2-based synthesis of N-carboxyanhydrides. Amino alcohol 1a was selected as the model compound for its challenging tertiary alcohol. An initial experiment produced traces of product 2a when T3P was added in one portion to a homogeneous mixture of Et3N and superbase DBU. The latter was included for its well-known propensity to promote the reactivity of amines with CO2. Gratifyingly, the yield of 2a improved when T3P was added slowly over 4 h (Table , entry 1). An increased amount of DBU (entry 2) or heterogeneous Cs2CO3 both improved the yield of 2a (entry 3). Counterintuitively, combining DBU and Cs2CO3 led to a decrease in yield (entry 4). This effect persisted when DBU was lowered to 50 mol % (entry 5), but lowering to 20 mol % did improve the overall yield (entry 6). Increasing the amount of T3P gave the best yield (entry 7). Control experiments showed that removing DBU decreased the yield (entry 8). Increasing the amount of DBU was detrimental (entry 9), as was excluding the CO2 atmosphere (entry 10).
1. Optimization Studies .
| Entry | Base (equiv) | T3P (equiv) | Yield (%) |
|---|---|---|---|
| 1 | Et3N (3.0) + DBU (1.0) | 1.3 | 25 |
| 2 | DBU (2.0) | 1.3 | 49 |
| 3 | Cs2CO3 (3.0) | 1.3 | 63 |
| 4 | Cs2CO3 (3.0) + DBU (1.0) | 1.3 | 59 |
| 5 | Cs2CO3 (3.0) + DBU (0.5) | 1.3 | 56 |
| 6 | Cs2CO3 (3.0) + DBU (0.2) | 1.3 | 76 |
| 7 | Cs2CO3 (3.0) + DBU (0.2) | 2.0 | 100 |
| 8 | Cs2CO3 (3.0) | 2.0 | 86 |
| 9 | Cs2CO3 (3.0) + DBU (1.0) | 2.0 | 68 |
| 10 | Cs2CO3 (3.0) + DBU (0.2) | 2.0 | <5 |
Yield (0.5 mmol scale) determined as the normalized GC-MS area of product 2a in relation to mesitylene (1 equiv).
Under Ar instead of a CO2 atmosphere.
With the optimized conditions in hand, we explored the substrate scope (Scheme ). 2-Oxazolidinones 2a–2e were obtained in good yields, with 2c isolated as a single diasteromer. In the case of aniline 1d, a slower addition of T3P over 12 h was found to improve the yield.
2. Substrate Scope of T3P-Mediated Cyclization of Amino Alcohols and CO2 .
a Isolated yields on 4.0 mmol scale.
b T3P added over 12 h.
c Dual addition.
d Racemic, (1S, 7R)-enantiomer is shown.
Proceeding to six-membered cyclic carbamates, we initially obtained only traces of 2f, despite extensive reoptimization (SI section 5.1). It was observed that the solution turned cloudy and the finely dispersed Cs2CO3 powder formed hard lumps when the 1,3-amino alcohol 1f was added, irrespectively of the reagent addition order. Lumping did not occur for the studied 1,2-amino alcohols 1a–1e. We hypothesized that when 1f reacts with CO2, the forming intermediate, likely cesium carbamate, precipitates out and causes lumping. Indeed, when 1,3-amino alcohols 1g–1l bearing lipophilic substituents were surveyed, the corresponding carbamates 2g–2l were obtained in good yields. Adding T3P over 12 h was beneficial, likely due to the low solubility of 1g–1l derived carbamates in solution. Under standard conditions, 2j was obtained in only 30% yield, likely due to cyclopropane decomposition. Gratifyingly, high dilution conditions using dual addition of 1j and T3P increased the yield of 2j to 45%.
Finally, we studied exotic seven-membered cyclic carbamates. Unsubstituted 2m was not observed, whereas substituted carbamates 2n–2p were obtained in modest to good yields. No urea side products were observed, inherent to the CDI-based method. To benchmark our results, standard CDI-mediated synthesis of products 2m–2o was attempted, but the yields were significantly reduced. This underlines that our CO2-based method exceeds the synthetic state-of-the-art.
The T3P-mediated dehydration is remarkably clean, with only minor impurities that can in most cases be removed by trituration or by filtering the crude product through a plug of silica. In contrast, a myriad of side-products is observed by GC-MS when TsCl is used as the dehydrating agent. Using TsCl instead of T3P resulted in lower yields of 2b and 2c. In this regard, T3P has significant advantages over TsCl. ,,
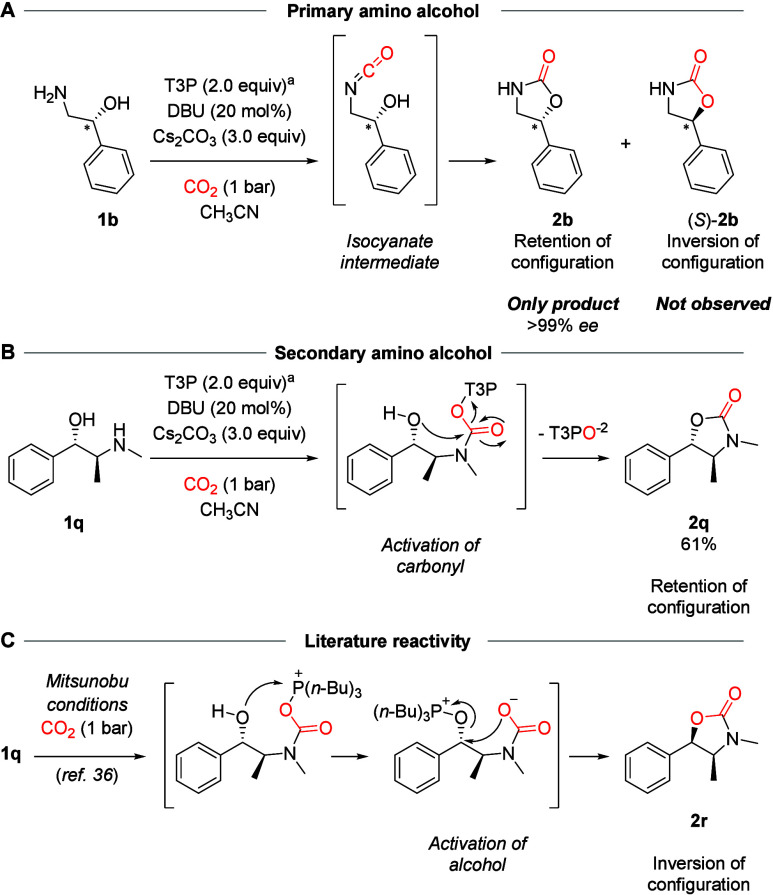
It was next studied whether the reaction mechanism proceeded via retention or inversion of configuration (Scheme A). Using enantiopure amino alcohol 1b, cyclized product 2b was analyzed by chiral HPLC and found to be >99 ee with retention of configuration. The absence of stereoinversion is consistent with an isocyanate intermediate. Although N-unsubstituted carbamates were our target, we were curious how N-methylated 1q would behave. We observed 2q as the exclusive diastereomer (Scheme B). Complete retention was surprising to us, since inversion product 2r was the expected product under the previously reported Mitsunobu conditions (Scheme C). Our results suggest that T3P is a potent activator of the carbamate group, leading to rapid dehydration (Scheme A) or nucleophilic attack (Scheme B).
3. Studies on the Stereochemical Outcome of the Reaction.
a T3P added over 4 h.
The role of DBU was next studied by in situ NMR using amino alcohol 1h, Cs2CO3, and mesitylene as an internal standard (SI section 2.1). Under CO2 without DBU, only 11% of carboxylated species (carbamate anion 3h) were observed in solution. In contrast, with 20 mol % DBU present, carboxylated species were present in 49%. These experiments suggest DBU has a bifunctional role in shifting the solution equilibrium from unreacted 1h to the carbamate salt 3h, and resolubilizing precipitated 3h.
Having studied solution behavior, we turned to analyzing the solid phase by FTIR (SI section 3.1). No peaks characteristic of ammonium species (RNH3 +) were observed, suggesting the Cs+ salt of 3h as the major species.
Based on the above-mentioned observations, we propose the following mechanism (Scheme ). In the presence of CO2, amino alcohol 1h rapidly forms carbamic acid 3h′. This species partially reacts with Cs2CO3 and precipitates out as salt A. , In a separate pathway, 3h′ forms a salt with DBU, which further reacts with salt A, forming well-soluble dimer B. This species is stabilized by coordination to Cs+ and hydrogen bonds between anionic 3h and protonated DBU. Similar dimers are known to be stabilized by coordination to Cs+ or a protonated superbase. The catalytic cycle begins with dimer B reacting with T3P, releasing salt A and forming carbamyl phosphate C. Base-mediated elimination forms isocyanate 2h′ and the mixed DBU/Cs salt D. Isocyanate 2h′ cyclizes to product 2h. The DBU/Cs salt D undergoes anion exchange with two equivalents of salt A. The first equivalent precipitates less soluble Cs2T3PO and the second equivalent regenerates dimer B. Overall, it seems the reaction between dimer B and T3P is the rate-determining step, since it was necessary to slowly add T3P. Adding all T3P at once led to lower yields. Presumably this was due to slow carbonate-mediated decomposition of T3P, a known reactivity of oxygen-nucleophiles. The influence of amino alcohol structural features on the reaction outcome is further discussed in SI section 1.1.
4. Proposed Mechanism, Where a DBU-Stabilized Carbamate Anion Dimer (B) is Dehydrated by T3P to an Isocyanate.
To conclude, we have developed a CO2-based method to synthesize N-unsubstituted five-, six-, and seven-membered cyclic carbamates, including bicyclic systems. The reaction is very clean, stereospecific, and high yielding, even when using amino alcohols with sterically demanding tertiary alcohols. Rare seven-membered cyclic carbamates are obtained in low-to-moderate yields, yet which are superior to state-of-the-art CDI-based syntheses. Mechanistic work indicates that the in situ formed carbamate salts are dehydrated to isocyanates using T3P. Catalytic DBU has a bifunctional role in shifting the solution equilibrium to more reactive and soluble species.
Supplementary Material
Acknowledgments
This work has been supported by NordForsk (Grant No. 85378) and the members of the Nordic Consortium for CO2 Conversion (NordCO2), and Academy of Finland (project 310767). J.K.M. is grateful for generous funding from the Väisälä Fund, the Orion Research Foundation, the Eemil Aaltonen Foundation, the Magnus Ehrnrooth Foundation, and the Funds of Nylands Nation.
The data underlying this study are available in the published article and its Supporting Information.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.5c01410.
The electronic Supporting Information provides general experimental information, in situ NMR experiments, FTIR spectra, experimental procedures, compound characterization, chiral HPLC results, and NMR spectra (PDF)
The authors declare no competing financial interest.
References
- Heravi M. M., Zadsirjan V.. Prescribed Drugs Containing Nitrogen Heterocycles: An Overview. RSC Adv. 2020;10(72):44247–44311. doi: 10.1039/D0RA09198G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberth C.. Heterocyclic Chemistry in Crop Protection. Pest Manag Sci. 2013;69(10):1106–1114. doi: 10.1002/ps.3615. [DOI] [PubMed] [Google Scholar]
- Vitaku E., Smith D. T., Njardarson J. T.. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014;57(24):10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Brindisi M.. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015;58(7):2895–2940. doi: 10.1021/jm501371s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacondio F., Silva C., Mor M., Testa B.. Qualitative Structure-Metabolism Relationships in the Hydrolysis of Carbamates. Drug Metab Rev. 2010;42(4):551–589. doi: 10.3109/03602531003745960. [DOI] [PubMed] [Google Scholar]
- Brooks C. A., Barton L. S., Behm D. J., Eidam H. S., Fox R. M., Hammond M., Hoang T. H., Holt D. A., Hilfiker M. A., Lawhorn B. G., Patterson J. R., Stoy P., Roethke T. J., Ye G., Zhao S., Thorneloe K. S., Goodman K. B., Cheung M.. Discovery of GSK2798745: A Clinical Candidate for Inhibition of Transient Receptor Potential Vanilloid 4 (TRPV4) ACS Med. Chem. Lett. 2019;10(8):1228–1233. doi: 10.1021/acsmedchemlett.9b00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M. E., Parsons R. L., Radesca L. A., Lo Y. S., Silverman S., Moore J. R., Islam Q., Choudhury A., Fortunak J. M. D., Nguyen D., Luo C., Morgan S. J., Davis W. P., Confalone P. N., Chen C. Y., Tillyer R. D., Frey L., Tan L., Xu F., Zhao D., Thompson A. S., Corley E. G., Grabowski E. J. J., Reamer R., Reider P. J.. Practical Asymmetric Synthesis of Efavirenz (DMP 266), an HIV-1 Reverse Transcriptase Inhibitor. J. Org. Chem. 1998;63(23):8536–8543. doi: 10.1021/jo981170l. [DOI] [Google Scholar]
- Xiao, D. ; Palani, A. ; Wang, C. ; Tsui, H.-C. ; Shih, N.-Y. ; Reichard, G. A. . Fused Ring NK1 Antagonists, US7041682B2; USA, 2005.
- Smith C. J., Ali A., Hammond M. L., Li H., Lu Z., Napolitano J., Taylor G. E., Thompson C. F., Anderson M. S., Chen Y., Eveland S. S., Guo Q., Hyland S. A., Milot D. P., Sparrow C. P., Wright S. D., Cumiskey A. M., Latham M., Peterson L. B., Rosa R., Pivnichny J. V., Tong X., Xu S. S., Sinclair P. J.. Biphenyl-Substituted Oxazolidinones as Cholesteryl Ester Transfer Protein Inhibitors: Modifications of the Oxazolidinone Ring Leading to the Discovery of Anacetrapib. J. Med. Chem. 2011;54(13):4880–4895. doi: 10.1021/jm200484c. [DOI] [PubMed] [Google Scholar]
- Ager D. J., Prakash I., Schaad D. R.. 1,2-Amino Alcohols and Their Heterocyclic Derivatives as Chiral Auxiliaries in Asymmetric Synthesis. Chem. Rev. 1996;96(2):835–875. doi: 10.1021/cr9500038. [DOI] [PubMed] [Google Scholar]
- Kochi T., Tang T. P., Ellman J. A.. Asymmetric Synthesis of Syn- and Anti-1,3-Amino Alcohols. J. Am. Chem. Soc. 2002;124(23):6518–6519. doi: 10.1021/ja026292g. [DOI] [PubMed] [Google Scholar]
- Niemi T., Fernández I., Steadman B., Mannisto J. K., Repo T.. Carbon Dioxide-Based Facile Synthesis of Cyclic Carbamates from Amino Alcohols. Chem. Commun. 2018;54(25):3166–3169. doi: 10.1039/C8CC00636A. [DOI] [PubMed] [Google Scholar]
- Mannisto J. K., Pavlovic L., Tiainen T., Nieger M., Sahari A., Hopmann K. H., Repo T.. Mechanistic Insights into Carbamate Formation from CO 2 and Amines: The Role of Guanidine–CO 2 Adducts. Catal. Sci. Technol. 2021;11(20):6877–6886. doi: 10.1039/D1CY01433A. [DOI] [Google Scholar]
- Díaz D. J., Hylton K. G., McElwee-White L.. Selective Catalytic Oxidative Carbonylation of Amino Alcohols to Ureas. J. Org. Chem. 2006;71(2):734–738. doi: 10.1021/jo0521908. [DOI] [PubMed] [Google Scholar]
- Gabriele B., Della Ca’ N., Mancuso R., Veltri L., Ziccarelli I.. Amidine– and Guanidine–based Synthetic Methods for CO2 Capture and Utilization. Curr. Opin Green Sustain Chem. 2023;41:100793. doi: 10.1016/j.cogsc.2023.100793. [DOI] [Google Scholar]
- Kleij A. W.. Advancing Halide-Free Catalytic Synthesis of CO2-Based Heterocycles. Curr. Opin Green Sustain Chem. 2020;24:72–81. doi: 10.1016/j.cogsc.2020.04.002. [DOI] [Google Scholar]
- Qin Y., Cauwenbergh R., Pradhan S., Maiti R., Franck P., Das S.. Straightforward Synthesis of Functionalized γ-Lactams Using Impure CO2 Stream as the Carbon Source. Nat. Commun. 2023;14(1):7604. doi: 10.1038/s41467-023-43289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo P. K., Zhang Y., Das S.. CO2-Promoted Reactions: An Emerging Concept for the Synthesis of Fine Chemicals and Pharmaceuticals. ACS Catal. 2021;11(6):3414–3442. doi: 10.1021/acscatal.0c05681. [DOI] [Google Scholar]
- Ion A., Van Doorslaer C., Parvulescu V., Jacobs P., De Vos D.. Green Synthesis of Carbamates from CO2, Amines and Alcohols. Green Chem. 2008;10(1):111–116. doi: 10.1039/B711197E. [DOI] [Google Scholar]
- Schilling W., Das S.. CO2-Catalyzed/Promoted Transformation of Organic Functional Groups. Tetrahedron Lett. 2018;59:3821–3828. doi: 10.1016/j.tetlet.2018.08.033. [DOI] [Google Scholar]
- Schilling W., Das S.. Transition Metal-Free Synthesis of Carbamates Using CO 2 as the Carbon Source. ChemSusChem. 2020;13:6246. doi: 10.1002/cssc.202002073. [DOI] [PubMed] [Google Scholar]
- Yu B., He L. N.. Upgrading Carbon Dioxide by Incorporation into Heterocycles. ChemSusChem. 2015;8(1):52–62. doi: 10.1002/cssc.201402837. [DOI] [PubMed] [Google Scholar]
- Wang L., Qi C., Xiong W., Jiang H.. Recent Advances in Fixation of CO2 into Organic Carbamates through Multicomponent Reaction Strategies. Chin. J. Catal. 2022;43(7):1598–1617. doi: 10.1016/S1872-2067(21)64029-9. [DOI] [Google Scholar]
- Wang W., Fu Y., Li Y., Yao R., Liu L., Chang W., Li J.. Ag(i)-Catalyzed Solvent-Free CO2 Capture with Homopropargylic Amines: An Efficient Access to 1,3-Oxazinan-2-Ones. Org. Chem. Front. 2018;5(22):3331–3335. doi: 10.1039/C8QO00978C. [DOI] [Google Scholar]
- Brunel P., Monot J., Kefalidis C. E., Maron L., Martin-Vaca B., Bourissou D.. Valorization of CO2: Preparation of 2-Oxazolidinones by Metal-Ligand Cooperative Catalysis with SCS Indenediide Pd Complexes. ACS Catal. 2017;7(4):2652–2660. doi: 10.1021/acscatal.7b00209. [DOI] [Google Scholar]
- Sun S., Zhou C., Yu J. T., Cheng J.. Visible-Light-Driven Palladium-Catalyzed Oxy-Alkylation of 2-(1-Arylvinyl)Anilines by Unactivated Alkyl Bromides and CO2: Multicomponent Reactions toward 1,4-Dihydro-2 H-3,1-Benzoxazin-2-Ones. Org. Lett. 2019;21(16):6579–6583. doi: 10.1021/acs.orglett.9b02700. [DOI] [PubMed] [Google Scholar]
- Xiong H., Wu X., Wang H., Sun S., Yu J. T., Cheng J.. The Reaction of O-Aminoacetophenone N-Tosylhydrazone and CO2 toward 1,4-Dihydro-2H-3,1-Benzoxazin-2-Ones. Adv. Synth Catal. 2019;361(15):3538–3542. doi: 10.1002/adsc.201900341. [DOI] [Google Scholar]
- Takeda Y., Okumura S., Tone S., Sasaki I., Minakata S.. Cyclizative Atmospheric CO 2 Fixation by Unsaturated Amines with T-BuOI Leading to Cyclic Carbamates. Org. Lett. 2012;14(18):4874–4877. doi: 10.1021/ol302201q. [DOI] [PubMed] [Google Scholar]
- Veltri L., Amuso R., Vitale P., Chiacchio M. A., Benincasa C., Gabriele B.. Synthesis of 1,3-Oxazine-2,4-Diones by DBU-Catalyzed Incorporation of Carbon Dioxide into 3-Ynamides. J. CO2 Util. 2021;52:101695. doi: 10.1016/j.jcou.2021.101695. [DOI] [Google Scholar]
- Li X., Benet-Buchholz J., Escudero-Adán E. C., Kleij A. W.. Silver-Mediated Cascade Synthesis of Functionalized 1,4-Dihydro-2H-Benzo-1,3-Oxazin-2-Ones from Carbon Dioxide. Angew. Chem., Int. Ed. 2023;62(11):e202217803. doi: 10.1002/anie.202217803. [DOI] [PubMed] [Google Scholar]
- Niemi T., Perea-Buceta J. E., Fernández I., Hiltunen O. M., Salo V., Rautiainen S., Räisänen M. T., Repo T.. A One-Pot Synthesis of N-Aryl-2-Oxazolidinones and Cyclic Urethanes by the Lewis Base Catalyzed Fixation of Carbon Dioxide into Anilines and Bromoalkanes. Chem. Eur. J. 2016;22(30):10355–10359. doi: 10.1002/chem.201602338. [DOI] [PubMed] [Google Scholar]
- Shi W., Benet-Buchholz J., Kleij A. W.. Catalytic Transformation of Carbon Dioxide into Seven-Membered Heterocycles and Their Domino Transformation into Bicyclic Oxazolidinones. Nat. Commun. 2025;16(1):1372. doi: 10.1038/s41467-025-56681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agami C., Couty F., Hamon L., Venier O.. Chiral Oxazolidinones from N-Boc Derivatives of β-Amino Alcohols. Effect of a N-Methyl Substituent on Reactivity and Stereoselectivity. Tetrahedron Lett. 1993;34(28):4509–4512. doi: 10.1016/0040-4039(93)88071-P. [DOI] [Google Scholar]
- Kaneti J., Kirby A. J., Koedjikov A. H., Pojarlieff I. G.. Thorpe-Ingold Effects in Cyclizations to Five-Membered and Six-Membered Rings Containing Planar Segments. The Rearrangement of N(1)-Alkyl-Substituted Dihydroorotic Acids to Hydantoinacetic Acids in Base. Org. Biomol Chem. 2004;2(7):1098–1103. doi: 10.1039/B400248B. [DOI] [PubMed] [Google Scholar]
- Paz J., Pérez-Balado C., Iglesias B., Muñoz L.. Carbon Dioxide as a Carbonylating Agent in the Synthesis of 2-Oxazolidinones, 2-Oxazinones, and Cyclic Ureas: Scope and Limitations. J. Org. Chem. 2010;75(9):3037–3046. doi: 10.1021/jo100268n. [DOI] [PubMed] [Google Scholar]
- Dinsmore C. J., Mercer S. P.. Carboxylation and Mitsunobu Reaction of Amines to Give Carbamates: Retention vs Inversion of Configuration Is Substituent-Dependent. Org. Lett. 2004;6(17):2885–2888. doi: 10.1021/ol0491080. [DOI] [PubMed] [Google Scholar]
- Magano J.. Large-Scale Amidations in Process Chemistry: Practical Considerations for Reagent Selection and Reaction Execution. Org. Process Res. Dev. 2022;26(6):1562–1689. doi: 10.1021/acs.oprd.2c00005. [DOI] [Google Scholar]
- Tran T. V., Shen Y., Nguyen H. D., Deng S., Roshandel H., Cooper M. M., Watson J. R., Byers J. A., Diaconescu P. L., Do L. H.. N-Carboxyanhydrides Directly from Amino Acids and Carbon Dioxide and Their Tandem Reactions to Therapeutic Alkaloids. Green Chem. 2022;24(23):9245–9252. doi: 10.1039/D2GC03507C. [DOI] [Google Scholar]
- McGuire T. M., López-Vidal E. M., Gregory G. L., Buchard A.. Synthesis of 5- to 8-Membered Cyclic Carbonates from Diols and CO2: A One-Step, Atmospheric Pressure and Ambient Temperature Procedure. J. CO2 Util. 2018;27:283–288. doi: 10.1016/j.jcou.2018.08.009. [DOI] [Google Scholar]
- Hedrick J. L., Piunova V., Park N. H., Erdmann T., Arrechea P. L.. Simple and Efficient Synthesis of Functionalized Cyclic Carbonate Monomers Using Carbon Dioxide. ACS Macro Lett. 2022;11(3):368–375. doi: 10.1021/acsmacrolett.2c00060. [DOI] [PubMed] [Google Scholar]
- Kortunov P. V., Siskin M., Baugh L. S., Calabro D. C.. In Situ Nuclear Magnetic Resonance Mechanistic Studies of Carbon Dioxide Reactions with Liquid Amines in Non-Aqueous Systems: Evidence for the Formation of Carbamic Acids and Zwitterionic Species. Energy Fuels. 2015;29(9):5940–5966. doi: 10.1021/acs.energyfuels.5b00985. [DOI] [Google Scholar]
- Mannisto J. K., Sahari A., Lagerblom K., Niemi T., Nieger M., Sztanó G., Repo T.. One-Step Synthesis of 3,4-Disubstituted 2-Oxazolidinones by Base-Catalyzed CO 2 Fixation and Aza-Michael Addition. Chem. Eur. J. 2019;25(44):10284–10289. doi: 10.1002/chem.201902451. [DOI] [PubMed] [Google Scholar]
- Mannisto J. K., Pavlovic L., Heikkinen J., Tiainen T., Sahari A., Maier N. M., Rissanen K., Nieger M., Hopmann K. H., Repo T.. N-Heteroaryl Carbamates from Carbon Dioxide via Chemoselective Superbase Catalysis: Substrate Scope and Mechanistic Investigation. ACS Catal. 2023;13(17):11509–11521. doi: 10.1021/acscatal.3c02362. [DOI] [Google Scholar]
- Davidson A., Foley D. A., Frericks-Schmidt H., Ruggeri S. G., Herman M., Lacasse S., Liu Y., McInturff E. L., Morris R., Mugheirbi N., Samas B., Sarkar A., Singer R. A., Witkos F., Yu S.. PH-Dependent Degradation of T3P-Related Byproducts. Org. Process Res. Dev. 2021;25(3):621–626. doi: 10.1021/acs.oprd.0c00431. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.