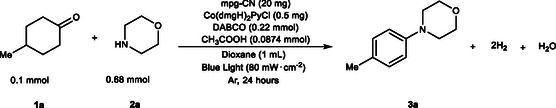
Table 1.
Screening of the reaction conditions of aniline synthesis.
| ||
|---|---|---|
| Entry | Deviations from standard conditions | Yield [%] |
| 1 | Noneb) | 49a) |
| 2 | Na‐PHI | 9 |
| 3 | Without mpg‐CN | n.d. |
| 4 | Without Co(dmgH)2PyCl | n.d. |
| 5 | In the dark | n.d. |
| 6 | mpg‐CN; Purple LEDc) | 16 |
| 7 | mpg‐CN; UV LEDd) | 27 |
| 8 | mpg‐CN; Co(dmgH)2PyCl (0.0024 mmol) | 48 |
| 9 | mpg‐CN; Co(dmgH)2PyCl (0.0048 mmol) | 50 |
| 10 | mpg‐CN (30 mg) | 44 |
| 11 | mpg‐CN (10 mg) | 46 |
| 12 | mpg‐CN (5 mg) | 32 |
| 13 | mpg‐CN; air | 28 |
| 14 | mpg‐CN; without DABCO | 10 |
| 15 | mpg‐CN; without AcOH | 30 |
GC yield;
Reaction conditions: ketone (0.1 mmol, 0.125 mL), morpholine (0.68 mmol, 0.59 mL), mpg‐CN 20 mg, Co(dmgH)2PyCl (0.0012 mmol, 0.5 mg), DABCO (0.22 mmol, 25 mg), glacial AcOH (0.0874 mmol, 0.005 mL), dioxane (1 mL), blue LEDs (λ max = 460 nm, 80 mW cm−2), 25 °C, 24 h, n.d. – not detected;
λ max = 400 nm, 60 mW cm−2;
λ max = 365 nm, 60 mW cm−2.
