Abstract
Background
Craniomaxillofacial sutures play a critical role in craniomaxillofacial development through continuous bone reconstruction and regeneration, processes modulated by mechanical tension. Bone suture stem cells (SuSCs) are central to these functions. Distraction osteogenesis, which promotes craniomaxillofacial suture growth, is a common therapeutic approach for craniofacial deformities. However, the underlying mechanisms by which mechanical forces drive suture and bone remodeling remain poorly understood, posing significant clinical challenges.
Methods
To investigate these mechanisms, we established a rapid maxillary expansion (RME) model in mice to widen the midpalatal suture. Single-cell RNA sequencing (scRNA-seq) was employed to identify subsets of SuSCs responsive to mechanical tension and analyze their differentiation potential under varying conditions. Further functional studies were conducted to explore the role of DALR anticodon binding domain containing 3 (Dalrd3) and its associated tRNA 3-methylcytosine (m3C) modification in SuSCs under mechanical tension.
Results
Our study identified a subset of SuSCs with multidirectional differentiation potential that shifted from a chondrogenic to an osteogenic trajectory in response to mechanical tension. Mechanical tension also upregulated Dalrd3 expression and its associated tRNA m3C modification in activated SuSCs. Knockdown of Dalrd3 in SuSCs significantly impaired osteogenic differentiation, proliferation, migratory capacity, and translational activity within the bone morphogenetic protein (BMP) signaling pathway. Furthermore, Dalrd3 knockdown suppressed the translational activity of inhibitor of DNA binding 3 (Id3), a key BMP-induced mediator of osteoblastogenesis. Restoring Id3 expression in Dalrd3-deficient SuSCs rescued their osteogenic, proliferative, and migratory functions.
Conclusions
These findings reveal a translational regulatory mechanism in SuSCs activated by mechanical tension and underscore the pivotal role of Dalrd3 in suture remodeling and bone formation. The insights provided by this study have the potential to guide targeted therapeutic strategies for optimizing distraction osteogenesis and other treatments for craniofacial deformities.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-025-04380-9.
Keywords: Bone suture stem cells, Mechanical tension, Dalrd3, Osteogenesis, TRNA m3 C modification
Background
Craniomaxillofacial growth and development are shaped by genetic, epigenetic, and environmental factors, particularly mechanical forces [1]. Craniomaxillofacial sutures protect surrounding tissues by absorbing mechanical stress and respond to orthopedic and orthodontic treatments by promoting distractive osteogenesis to correct craniofacial deformities [2, 3]. For instance, rapid maxillary expansion (RME) addresses maxillary transverse deficiency, corrects maxillary hypoplasia, and alleviates pediatric obstructive sleep apnea [4]. Despite various attempts to enhance bone remodeling post-suture expansion through pharmaceutical, physical, and genetic methods, high recurrence rates remain, especially in severe congenital conditions like cleft lip and palate [5–8]. Hence, it is critical to further understand the underlying mechanism of how suture reacts to mechanical forces and remodels the craniofacial deformities.
The craniomaxillofacial suture presents a narrow and complex structure. Notably, mice and humans share significant similarities in craniomaxillofacial growth [9]. Hou et al. have found that expansion forces applied to the midpalatal suture stimulate bone and cartilage formation by enhancing mesenchymal cell proliferation and differentiation in mouse models [10]. However, suture mesenchymal cells consist of various subpopulations rather than being homogeneous. Indeed, single-cell RNA sequencing (scRNA-seq) study has identified that multiple types of mesenchymal cells in the midpalatal suture, such as pericytes, osteoblastic and chondrogenic cells [9]. Furthermore, studies have found that Gli1+ or Prx1/Prrx1+ craniomaxillofacial suture stem cells (SuSCs) contributed to mechanical force-induced osteogenesis [11–14]. However, the exact mechanism of how mechanical forces induce the osteogenic properties of SuSCs requires further investigation.
Here, we established a midpalatal suture RME model in mice and found a high degree of heterogeneity among mesenchymal cells in the midpalatal suture, including a subset of SuSCs with pluripotent properties. These SuSCs poised to chondrogenic differentiation during homeostasis stage but switched to osteogenic differentiation when exposed to mechanical tension. In addition, our scRNA-seq data revealed that mechanical tension elevated DALR anticodon binding domain containing 3 (Dalrd3) levels in SuSCs, enhancing the translation of the inhibitor of DNA binding 3 (Id3) mRNA, a central component of bone morphogenetic protein (BMP) signaling pathway, by modulating tRNA 3-methylcytosine (m3 C) modification, thereby promoting osteogenic differentiation under mechanical tension. Thus, this research demonstrated that mechanical tension not only influence transcriptional activity but also boost the translational capacity of SuSCs to foster bone suture osteogenesis.
Materials and methods
The establishment of RME models
Seven‐week‐old male C57BL/6 mice were utilized to establish a model of RME as previously described [6, 7, 11]. In brief, micewere anesthetized with 1% pentobarbitonar intraperitoneal injection (50 mg/kg, Sigma-Aldrich). 0.014-inch stainless steel orthodontic wire (GAC International Inc.), we created an opening loop, which was then bonded to the first and second maxillary molars on both sides with a light-cured adhesive (3 M Unitek). This setup produced an initial force of 0.5 N. For the sham operation, non-functional opening loops were prepared without any expansion force and similarly bonded to the first and second maxillary molars. Mice were euthanized with CO2 inhalation at different time points—3, 7, 14, 21, or 28 days—consisting of six mice in each group for each time interval. Then maxilla specimens were collected from euthanized mice for subsequent micro-CT scanning and histological staining. A total of 78 C57BL/6 mice were included in the study. Healthy mice, displaying no visible abnormalities in appearance or behavior, were randomly assigned to groups using a random number generator. Treatment and measurement sequences for each mouse were also randomized. Group allocation details were disclosed during the outcome evaluation and data analysis stages. Throughout the experiment, the body weight, appearance, and behavior of the mice were monitored weekly to ensure their well-being. Our reporting of animal experiments adheres to the ARRIVE guidelines.
Micro-CT and histomorphometric analyses
Micro-CT analysis was conducted in accordance with established guidelines for assessing rodent bone microstructure [15]. The harvested maxilla specimens were fixed in 4% polyoxymethylene (Beyotime) for two days and scanned using a micro-computed tomography device (micro-CT50, Scanco Medical) with a spatial resolution of 10 μm at 70 kV and 200 μA. After scanning, three-dimensional reconstructions were created, and the microarchitecture and bone parameters of the midpalatal suture were evaluated using Materialise's interactive medical image control system 21.0 (MIMICS, Materialise). The regions of interest (ROIs) were defined as follows: in the sagittal direction, there was a 20 μm area on each side at the junction of the first and second molars. In the coronal direction, the area extends 20 μm on each side from the center of the middle palatal suture. Bone mineral density (BMD) and bone volume fraction (BV/TV) were calculated within the ROIs.
Histological staining
The fixed specimens were decalcified by EDTA decalcifying solution (Solarbio Science & Technology) for a month, then dehydrated, embedded in paraffin, and serially sectioned at a 5 µm thickness. Paraffin sections were stained by hematoxylin–eosin (H&E) staining kit (Solarbio Science & Technology) and saffron-o and fast green (SOFG) stain kit (Solarbio Science & Technology) following the manufacturer’s protocol. For immunohistochemistry (IHC) staining, paraffin sections were incubated with Rabbit polyclonal antibodies to Id3(1:100, proteintech) and Dalrd3(1:100, Bioss). All results were panoramically scanned by digital pathology slide scanner (Kfbio). The IHC score was measured on a continuous scale from 0 to 300, calculated by multiplying the proportion of stained cells by the staining intensity (intensity score ranging from 0 to 3) [16].
Library preparation for droplet-based scRNA-seq
During sample preparation, eight male C57BL/6 mice, aged 7 weeks, were assigned to two groups: the RME group and the control group, with four mice in each. After 7 days of RME treatment, the cranial-maxillary complex was meticulously dissected under a stereo microscope to isolate tissue from the midpalatal suture region. In each group, two samples were combined to create a single-cell suspension. Tissue samples were minced into fragments smaller than 1 mm3 and incubated in 1 mg/mL collagenase type II (Sigma-Aldrich) at 37 °C for 40 min. After digestion, the mixture was filtered through a 40-μm filter, centrifuged, and the supernatant removed. To lyse red blood cells, 1 mL of ACK lysis buffer was added on ice for 2 min, followed by another round of centrifugation to remove the supernatant.
Libraries for scRNA-seq were constructed utilizing the DNBelab C Series Single-Cell Library Prep set (MGI, 1,000,021,082) [17]. Briefly, single-cell suspensions underwent a series of processes, including droplet encapsulation, emulsion disruption, bead collection for mRNA capture, reverse transcription, and subsequent cDNA amplification and purification to create barcoded libraries. Indexed sequencing libraries were assembled in accordance with the manufacturer's instructions, and their concentrations were assessed using a Qubit ssDNA Assay kit (Thermo Fisher Scientific). Ultimately, sequencing was performed on a MGISEQ2000 sequencer at the China National GeneBank (CNGB).
scRNA-seq data analysis
After obtaining the initial scRNA-seq data, raw reads were processed by DNBC4 tools (Version 2.0.7, https://github.com/MGI-tech-bioinformatics/DNBelab_C_Series_HT_scRNA-analysis-software) to obtain matrix data. The resulting expression matrix was imported into the Seurat package (Version 4.0) for further analysis. We excluded genes detected in fewer than three cells, as well as cells that expressed fewer than 200 or more than 5,000 genes, and those with mitochondrial content exceeding 10%. Doublets were identified using DoubletFinder, and any cells exhibiting markers characteristic of different cell types were eliminated from further analysis. Next, variable genes were selected using the Find Variable Genes function in Seurat and performed principal component analysis (PCA). Dimensionality reduction and visualization using uniform manifold approximation and projection (UMAP) were conducted based on the outcomes of the PCA analysis. We annotated the cell types of different subclusters based on specific genes expressed in each subgroup. To further recluster mesenchymal cells, the same strategy was applied using PCA, UMAP, and clustering.
Single-cell pseudotime trajectories were computed using the Monocle2 and Monocle3 R packages. Gene ontology (GO) enrichment analyses for each population were conducted using Gene Set Variation Analysis (GSVA) package with default parameters. We identified differentially expressed genes (DEGs) with the FindMarkersAll function. The top 50 DEGs from each cluster were subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) and GO pathway analysis using the KEGG Pathway Database (https://www.kegg.jp/kegg/pathway.html) and GO Pathway Database (Gene Ontology Resource). A p-value below 0.05 was deemed statistically significant. Additionally, DEGs of SuSCs between the RME and control groups were identified using the FindMarkersAll function. Genes with a false discovery rate (FDR) of less than 0.05 and an absolute log fold change (|logFC|) exceeding 0.585 were classified as DEGs. Genes with increased expression in SuSCs from the RME group were further analyzed using KEGG and GO pathway analysis.
Isolation, cultivation and multipotential differentiation of SuSCs
The procedure for isolating and cultivating SuSCs was adapted from earlier research studies [18, 19]. The midpalatal suture was carefully separated under a stereo microscope from one-day-old C57/BL6 mice, along with approximately 0.5 mm of adjacent palatal bone on each side. The suture was then divided into two pieces and placed in a T25 culture flask, where it was incubated in MEM alpha basic medium (Gibco) containing 10% FBS (Gibco) and 1% penicillin–streptomycin-amphotericin B Solution (Beyotime) at 37 °C and 5% CO2. After 7 days, primary cells were treated with 0.25% trypsin–EDTA (Gibco) and sub-cultivated. Cells from passages 2–3 were used for subsequent experiments.
Osteogenic, adipogenic, or chondrogenic differentiation were performed following established protocol [18]. For osteogenic induction, SuSCs were cultured with osteogenic differentiation medium (Procell). After seven days, alkaline phosphatase (ALP) staining was performed by BCIP/NBT solution (Beyotime). The staining results were visualized using a microscope (Leica) and quantified using integrated optical density (IOD) measurements. For Alizarin red staining (ARS), SuSCs were stained with alizarin red after 21 days of induction. The mineralized matrix was then dissolved using 10% cetylpyridinium chloride, and absorbance was measured at 562 nm using a microplate reader (Thermo Fisher Scientific) [20]. For adipogenic induction, SuSCs were treated with adipogenic medium (Cyagen) and stained with oil red O according to the manufacturer’s instructions. Chondrogenic differentiation was achieved by culturing SuSCs in chondrogenic differentiation medium (Cyagen), followed by staining with alcian blue as the manufacturer's protocol.
Flow cytometry analysis
Flow cytometry analysis was conducted following standard procedures as previously described [18]. Cultured SuSCs from passages p2 or p3 were digested into a cell suspension, centrifuged to remove the supernatant, and then resuspended in PBS. The cells were then stained with antibodies specific to CD45 (1:200, Thermo Fisher Scientific), CD11b (1:200, Thermo Fisher Scientific), CD44 (1:200, BD Biosciences), and Sca1 (1:200, BD Biosciences). The staining process took place at 25 °C for 20 min, protected from light, followed by a washing step with staining buffer. Data were acquired using the Attune NxT4 (Thermo Fisher Scientific) and analyzed with FlowJo software.
Application of mechanical stretch to SuSCs in vitro
Mechanical stretch was applied to SuSCs in vitro using the Flexcell Tension System (Fx5000, Flexcell International Corporation) following previous studies [8, 21–24]. Cells were seeded at a density of 1.5 × 104 cells/cm2 onto a collagen type I-coated 6-well BioFlex culture plate (Flexcell). After 24 h, a computer-controlled device was used to apply longitudinal stretching to the cells, incorporating a range of frequencies as mechanical parameters (0.0125 Hz, 0.025 Hz, 0.05 Hz, 0.1 Hz, 0.5 Hz, or 1 Hz) and a sinusoidal stretch magnitude (0.5%, 1%, 2%, 3%, 4%, or 5%). The stretch was applied for 4 h daily over a 3-day period. During this process, cells were maintained at 37 °C in an incubator with 5% CO2. Cells cultured under static conditions served as the control group (Ctrl). Cells were collected at the same time point for further analysis. For the ARS assay, SuSCs were subjected to mechanical stretching for 3 days and cultured in osteogenic differentiation medium for 7 days before being stained with Alizarin Red.
Immunofluorescence staining of F-actin and EdU
Cell morphology was assessed by staining with F-actin. SuSCs were fixed in 4% paraformaldehyde for 20 min, followed by a 10-min permeabilization with 0.1% Triton X-100 (Aladdin). The actin cytoskeleton was stained with Fluorescein Phalloidin (MCE) for 1 h at room temperature in the dark. Afterward, the cells were counterstained with DAPI (Solarbio Science & Technology) for 5 min. Images were captured using an inverted fluorescent microscope (Zeiss).
Cell proliferation was assessed using an EdU Cell Proliferation Kit (Beyotime) according to the manufacturer’s instructions. In brief, SuSCs were incubated with the EdU working solution for 4 h at room temperature, followed by fixation with 4% paraformaldehyde for 20 min and permeabilization with 0.1% TritonX-100. The cells were then treated with Click Additive Solution for 30 min in the dark, stained with DAPI for 5 min, and imaged with the fluorescent microscope.
Quantitative real‐time polymerase chain reaction (qRT-PCR)
After mechanical loading, total RNA was isolated from SuSCs using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. Reverse transcription was then performed using the HiScript III RT SuperMix for qPCR (Vazyme), and the resulting cDNA was subjected to RT-PCR analysis with ChamQ Universal SYBR qPCR Master Mix (Vazyme) on the StepOnePlus™ RT-PCR System (Applied Biosystems). The primer sequences for the target genes are provided in Table S1, with glyceraldehyde 3-phosphate dehydrogenase (Gapdh) utilized as the internal control. Relative expression levels of the mRNAs were calculated using the 2 − ΔΔCt method.
RNA interference
Dalrd3 knockdown in SuSCs was achieved by transfecting the cells with small interfering RNAs (siRNAs; see Table S1). SiRNA targeting Dalrd3 (siDalrd3, RiboBio) and scrambled siRNA (siNC) were introduced into cells with Lipofectamine® RNAiMAX (Invitrogen) following the manufacturer’s guidelines. The efficiency of siRNA transfection was evaluated through qRT-PCR and western blotting.
Western blotting
Western blotting was performed as described in our previous study [25]. Cells were lysed using radioimmunoprecipitation assay buffer (Beyotime) containing a protease inhibitor cocktail (Roche). Protein extracts were separated using a 10% sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE) and subsequently transferred to a polyvinylidene fluoride (PVDF) membrane. Following transfer, the membrane was blocked with 5% skimmed milk and incubated with the following primary antibodies: anti-Runx2 (1:1000, Affinity Biosciences), anti-Osterix (1:1000, Affinity Biosciences), anti-Dalrd3 (1:1000, Bioss), anti-Mettl2 (1:1000, proteintech), anti-Id3 (1:1000, proteintech), and anti-Gapdh (1:2000, Proteintech). Afterward, the membrane was treated with anti-rabbit secondary antibodies (1:5000, Proteintech). Protein detection was carried out using the Tanon 5200 Multi Intelligent Imaging System (Tianneng).
Northwestern blotting and Northern blotting
Northwestern blotting and Northern blotting were performed as previously described [25]. For Northwestern blotting, a total of 2 μg of RNA was combined with 2 × RNA loading buffer, denatured at 95 °C for 5 min, and then resolved via 15% urea-polyacrylamide gel electrophoresis in 1 × Tris–borate-EDTA (TBE) buffer (Solarbio Science & Technology). After electrophoresis, the RNA was transferred to a positively charged nylon membrane and crosslinked with UV light. It was then incubated overnight with anti-m3 C antibody (1:1000, Proteintech). For the Northern blotting procedure, U6 small nucleolar RNA (snoRNA) was analyzed using digoxigenin-labeled probes specific to U6 snoRNA (Sangon Biotech). The signals from the digoxigenin and anti-m3 C antibodies were detected with the Tanon 5200 Multi Intelligent Imaging System.
Cell proliferation and cell migration assay
Cell proliferation was assessed at scheduled time points using the Cell Counting Kit-8 (CCK-8) (Dojindo) according to the manufacturer’s instructions. SuSCs were seeded at a density of 1,000 cells per well in a 96-well plate (NEST) and cultured in complete medium for 6, 24, 48, and 72 h at 37℃. Following incubation, cells were treated with the CCK-8 solution for 2 h at 37℃ in the dark. Optical density (OD) was then measured at 450 nm using a microplate reader (Epoch2, Biotek).
In the migration assay, SuSCs (5 × 104 cells per well) were resuspended in 200 μL of MEM alpha medium lacking FBS and placed into a transwell insert (Corning). The lower chamber received 500 μL of MEM alpha with 10% FBS. After a 24-h period, cells that had passed through the pores were subjected to staining with Crystal Violet Staining Solution. The stained area was photographed, and the number of cells that migrated was counted under a microscope in five randomly chosen fields.
Ribosome nascent-chain complex-bound mRNA sequencing (RNC-seq) and data analysis
RNC-seq was conducted following previously established protocols [26]. In summary, siNC-SuSCs and siDalrd3-SuSCs were treated with 100 μg/mL cycloheximide for 15 min at 37 °C. The cells were then lysed using a cell lysis buffer composed of 1% Triton X-100 in a ribosome buffer containing 15 mM MgCl₂, 200 mM KCl, 20 mM HEPES–KOH (pH 7.4), 2 mM dithiothreitol, and 100 μg/mL cycloheximide. After lysing, the samples were centrifuged at 16,200 × g for 10 min at 4 °C, and 10% of the supernatant was set aside as an input control. The remaining lysate was carefully placed onto a sucrose buffer consisting of 30% sucrose in polysome extraction buffer and then subjected to ultracentrifugation at 174,900 × g for 5 h at 4 °C using a SW32 rotor (Beckman) to separate the RNC pellets containing polysome fractions. Afterward, RNC-mRNAs were isolated using TRIzol reagent.
The construction of the cDNA library and sequencing were carried out by the Beijing Genomics Institute using the BGISEQ-500 platform (BGI). The generated high-quality reads were aligned to the UCSC mouse genome (GRCm38/mm10) using Hisat2. The normalization of gene expression levels was carried out through the fragments per kilobase of transcript per million mapped reads (FPKM) method. To determine translation ratios (TRs), we applied the formula: TR = (FPKM in RNC-seq)/(FPKM in input RNA-seq). Genes were categorized as differentially translated if their FPKM values were above 0.01 and if there was a TR change of at least 0.5-fold.
Polysome profiling
Polysome profiling was performed following previously established protocols [27]. In brief, siNC-SuSCs and siDalrd3-SuSCs cells were exposed to 100 μg/mL cycloheximide for 15 min at 37 °C. The cells were then lysed in a polysome extraction buffer on ice for 10 min, followed by centrifugation at 13,000 × g for 10 min at 4 °C. After obtaining the supernatant (1 mL), it was gently placed onto a pre-formed 10%−50% sucrose gradient (11 mL) and subjected to centrifugation at 36,000 rpm for 3 h at 4 °C in an SW41 rotor (Beckman). The separated layers were processed using the BR-188 Density Gradient Fractionation System (Brandel) with a flow rate set to 0.75 mL/min, monitoring the absorbance at 254 nm. Once the polysomes were isolated, RNA was extracted for subsequent analysis via qRT-PCR.
Statistical analysis
Quantitative findings are reported as mean values accompanied by standard deviation (SD). Statistical evaluations were conducted using Student’s t-test or one-way analysis of variance (ANOVA), with significance defined as p < 0.05. The management of data and subsequent statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software).
Results
Establishment of murine RME model and changes in the midpalatal suture
We established a murine RME model and collected maxillary bones from mice after 3, 7, 14, 21, and 28 days post-expansion (Fig. 1a, b). Weight measurements indicated weight loss from days 1 to 3 due to feeding difficulties caused by the RME device. However, mice gradually regained weight as they adapted (Fig. S1). Micro-CT scans and subsequent 3D reconstruction revealed that, compared to the stable midpalatal sutures in the non-expansion (control) group, the suture width in the expansion (RME) group began to increase after 3 days. By day 7, new bone with a spiky appearance started to form along the suture edges, and by day 28, this new bone progressively filled the expanded suture, creating a new suture (Fig. 1c, d).
Fig. 1.
Histological and morphological changes in midpalatal sutures following expansion in mice. a Establishment timeline for RME model in mice. b Occlusal view of the mouse maxilla in different groups: control group (left), RME group with expansion device (center), and RME group without expansion device (right). Scale bar: 2 mm. c Horizontal plane views of three-dimensional micro-CT reconstructions of maxillae, highlighting the observed target area within a red box. d Three-dimensional micro-CT reconstructions of the palatal suture region in control and RME groups at various time points, with horizontal plane views (upper row) and coronal plane views (lower row). e Schematic illustration showing the measurement of suture width and palatal width on the coronal plane. f Micro-CT analysis comparing suture width, palatal width, BMD, and BV/TV ratios between control (n = 6) and RME groups (n = 6) over time. g H&E and SOFG staining of midpalatal sutures in the control group at various time points. Scale bar:50 μm. h H&E and SOFG staining of midpalatal sutures in the RME group at corresponding time points. Scale bar: 50 μm. *P < 0.05, **P < 0.01, ***P < 0.001. One-way or two-way ANOVA with Tukey’s multiple comparisons test among all groups was used
Micro-CT analysis showed significant increases in both suture width and palate width after 3 days of expansion. No significant difference in suture width was observed between expansion for 3 and 7 days, suggesting maximum expansion was achieved by day 3. After 14-day expansion, the suture width began to decrease, returning to control levels by day 28 due to new bone formation. The palate width remained stable from day 3 to day 28 post-expansion, significantly exceeding that of the control group (Fig. 1e, f). Subsequent measurements of bone parameters of ROIs revealed that BMD and BV/TV in the RME group were significantly lower than in the control group on day 3 post-expansion. As new bone formed within the expanded suture, BMD and BV/TV gradually increased, returning to control levels by day 28 post-expansion (Fig. 1f).
Histological analysis revealed that the control group's palatal suture primarily comprised chondrocytes and mesenchymal tissue, with the edges of the palatal bones covered by a thin layer of chondrocytes connected by fibrous tissue. No significant changes were observed in the control group (Fig. 1g). In the RME group, the fibrous tissue at the suture was stretched and sparse, with deformed and reduced chondrocytes at day 3. By day 7, mesenchymal cells began migrating into the suture from the oral and nasal sides, with early bone formation. By day 14, the suture was filled with mesenchymal tissue, and significant new bone formation was observed. By day 21, new bone increased significantly, and chondrocytes along the edges of the palatal bones disappeared. Until day 28, new bone formation closed the suture, creating a more interwoven structure with fewer chondrocytes compared to the non-expansion group (Fig. 1h). These histological changes align with previous research [10] and our Micro-CT analysis results.
Identification of pluripotent SuSCs among heterogeneous mesenchymal cells in the midpalatal suture
To identify and evaluate different cell populations in both expanded and non-expanded midpalatal sutures, we dissected the sutures from mice 7 days post-expansion and subjected them to enzymatic digestion for scRNA-seq analysis (Fig. 2a). This time point was selected because the sutures were actively responding to mechanical expansion and undergoing osteogenesis. After quality control to filter out low-quality cells, a total of 26,870 cells were obtained, with each cell expressing 28,126 genes. Unsupervised clustering was performed by UMAP for dimensionality reduction (Fig. 2b).
Fig. 2.
Single-cell atlas characterization of midpalatal suture in both control and RME groups. a Schematic overview of the sample preparation process for scRNA-seq analysis of the midpalatal suture. b UMAP visualization of scRNA-seq data from cells in both control and RME groups, showing distribution across seven distinct cell clusters. c Dot plot illustrating the specific expression levels of marker genes across various cell types. d UMAP atlas with projected expression levels of marker genes corresponding to the seven identified cell clusters. e UMAP plot showing the spatial distribution of seven mesenchymal cell subclusters. f Heatmaps displaying the top differentially expressed genes within each mesenchymal cell cluster, identified through unsupervised clustering. g Trajectory analysis of mesenchymal populations ordered by pseudotime values, illustrating progression across cell states
Cells were classified into seven subgroups based on classic cell surface markers: blood cells (Hemgn), mesenchymal cells (Bgn), endothelial cells (Pecam1), NKT cells (Cd3 d), myeloid cells (Cd14), epithelial cells (Krt14), and B cells (Cd79a) (Fig. 2c, d). Among these cells, mesenchymal cells (5,182 cells) were isolated for further clustering and marker analysis, which revealed seven distinct clusters of mesenchymal cells based on gene expression: SuSCs (Wnt5a, Ctsk, Prrx1), progenitors (Ogn, Aldh1a1), osteo-chondrogenic precursors (Mmp13, Aspn, Runx2), osteoblastic mesenchymal cells (Ifitm5, Smpd3, Mef2c), chondrogenic mesenchymal cells (Acan, Ucma, Col2a1), fibroblastic precursors (Pi16), and fibroblasts (Pdpn) (Fig. 2e, f).
Based on the gene expression dynamics of mesenchymal cells, pseudotime analyses revealed distinct differentiation trajectories within each cluster, indicating that the seven clusters were in various developmental states. SuSCs primarily occupied the upper branch in the pseudotime analysis, with the lowest pseudotime value, positioned at the initial point of the developmental tree, suggesting their role as the origin for other subclusters. Osteo-chondrogenic and fibroblastic differentiation occurred along separate branches (Figs. 2g and S2). This finding was supported by GO enrichment analysis for each cluster (Figs. 3a and S3). SuSCs showed upregulation of pathways related to Wnt signaling and response to mechanical stimuli KEGG enrichment analysis further indicated that SuSCs upregulated pathways regulating the pluripotency of stem cells (Fig. 3b). Progenitors also exhibited high expression of stemness-related genes (Fig. 3a, b). Additionally, osteo-chondrogenic precursors showed significant upregulation in the GO terms related to chondrocyte and osteoblast differentiation. Osteoblastic mesenchymal cells were associated with osteoblast differentiation and ossification, while chondrogenic mesenchymal cells were inclined towards cartilage development and chondrocyte differentiation (Fig. S3). Fibroblast-associated cells expressed genes related to elastic fiber assembly and fibroblast proliferation (Fig. S3).
Fig. 3.
Identification and stemness characteristics of SuSCs via scRNA-seq. a GO enrichment analysis highlighting the biological functions of SuSCs and progenitor cells. b KEGG pathway enrichment analysis of signaling pathways active in SuSCs and progenitors. c Violin plots showing the proportions of Wnt and Hedgehog pathway activity across seven cell clusters, analyzed by GSVA. d UMAP plot illustrating expression levels of stem cell marker genes within mesenchymal cells. e Quantitative analysis of mesenchymal cells expressing different stem cell marker genes. f Venn diagram depicting the homogeneity and heterogeneity of SuSCs, by counting the number of cells expressing Prrx1, Ctsk, Cd44, Ly6a, and Axin2 genes. **P < 0.01, ***P < 0.001. One-way ANOVA comparisons to SuSCs was used (c)
Furthermore, GSVA analysis indicated that SuSCs and progenitors had the highest scores for Wnt and Hedgehog signaling pathways (Fig. 3c). To further confirm the identification of SuSCs, we selected several mesenchymal stem cell markers along with five stem cell markers—Prrx1, Ctsk, Axin2, Ly6a, and Cd44—that were prominently expressed in SuSCs (Fig. 3d, e). By quantifying SuSCs co-expressing these stemness genes and those expressing each gene individually, we identified both homogeneity and heterogeneity among SuSCs populations with distinct stemness markers (Fig. 3f). Overall, we identified SuSCs as a pluripotent cell population responsive to mechanical expansion.
Expansion of the midpalatal suture induces osteogenic differentiation and Dalrd3 expression levels elevation of SuSCs
We investigated the impact of mechanical expansion on SuSCs in the midpalatal suture by conducting a pseudotime analysis, mapping the distribution of subclusters along the SuSCs trajectory in both expanded and non-expanded sutures. In non-expanded sutures, SuSCs exhibited a tendency to differentiate into chondrogenic mesenchymal cells during the natural growth state (Fig. 4a). When we mapped the expression of Col2a1, Acan, Ucma, and Sox9 onto the pseudotime trajectory, we observed a gradual increase in these markers, indicating a progression toward more differentiated chondrogenic mesenchymal cells, along the differentiation trajectory (Fig. 4b). Conversely, in expanded sutures, SuSCs showed a propensity to differentiate into osteoblastic mesenchymal cells (Fig. 4c). This was corroborated by the expression patterns of Runx2, Bglap2, Sp7, and Pth1r, markers of more differentiated osteoblastic mesenchymal cells, which also increased progressively along the differentiation trajectory (Fig. 4d).
Fig. 4.
scRNA-seq analysis of differentiation trajectories, and gene expression in SuSCs between control and RME groups. a Unbiased pseudotime trajectory analysis of mesenchymal cells in the control group, illustrating differentiation from undifferentiated SuSCs (purple) to differentiated chondrogenic and fibroblast cells (yellow). b Trajectory analysis identifying mesenchymal cells in the control group expressing key differentiation markers: Col2a1, Acan, Umca, and Sox9. c Unbiased pseudotime trajectory analysis of mesenchymal cells in the RME group, showing progression from undifferentiated SuSCs (purple) to differentiated osteoblastic and fibroblast mesenchymal cells (yellow). d Trajectory analysis identifying mesenchymal cells in the RME group expressing osteogenic markers: Runx2, Bglap2, Sp7, and Pth1r. e Comparative GSVA analysis of SuSCs between control and RME groups, highlighting differences in cell cycle activity, and Wnt and Hedgehog pathway proportions. f Volcano plot of differentially expressed genes in SuSCs between control and RME groups, with significantly upregulated genes in the RME group marked in red and downregulated genes in blue. g UMAP plot showing Dalrd3 expression levels in SuSCs from control and RME groups. h Trajectory analysis showing mesenchymal cells expressing Dalrd3 in control and RME groups. i GO and KEGG enrichment analysis of genes significantly upregulated in the RME group. Student’s t test was used
To further explore the impact of mechanical expansion on the proliferative capacity of SuSCs, we employed GSVA to score the cell cycle signaling pathway and quantified the expression levels of Mki67, Ccnd1, and Birc5 in cells from both expanded and non-expanded SuSCs. Our data indicated that SuSCs from expanded sutures demonstrated a greater proliferative capacity than those from non-expanded sutures (Figs. 4e and S4). Additionally, SuSCs from the expanded sutures exhibited enhanced stemness compared to their non-expanded counterparts (Fig. 4e).
Next, we utilized heat maps to explore differentially expressed genes and uncover the mechanisms underlying the differentiation differences between control and RME groups. We found that C230014O12Rik, Ufd1, and Dalrd3 were the top three genes with significantly higher expression in SuSCs of the RME group compared to the control group (Fig. 4f). We used UMAP plots to confirm that Dalrd3 expression levels in SuSCs increased after RME (Fig. 4g). Trajectory analysis also demonstrated the distribution of Dalrd3 in mesenchymal cells (Fig. 4h). GO and KEGG analyses revealed that the upregulated genes in SuSCs of the RME group were significantly enriched in signaling pathways related to protein translation (Fig. 4i). Recent studies have found that the directed differentiation of stem cells is tightly controlled by protein translation [28]. Through protein translation, tRNA modifications and abundance are emerging as important regulators of stem cell function [29]. Notably, DALRD3 and METTL2 form a complex that recognizes arginine tRNAs for m3 C modification. DALRD3 mutations in human cells result in loss of tRNA-Arg m3 C modification, resulting in developmental delays and early-onset epileptic encephalopathy [30]. Given its critical role in human development, it is essential to further explore the function of Dalrd3 in SuSCs differentiation in this study.
Inhibition of Dalrd3 in SuSCs reduces osteogenic differentiation
To explore the function of Dalrd3 in SuSCs, we first isolated SuSCs from midpalatal suture-derived explants and established an in vitro culture system of SuSCs. SuSCs exhibited typical stem cell morphology, characterized by spindle-shaped or irregular triangular forms, abundant cytoplasm, short cytoplasmic extensions, and consistent structures across various magnifications (Fig. S5a). Flow cytometry analysis of SuSCs showed high positivity for CD44 (91.09 ± 5.71%) and Sca-1 (98.40 ± 0.78%), but exhibiting low levels of the hematopoietic stem cell marker CD45 (4.08 ± 1.61%) and the myeloid cell marker CD11b (3.76 ± 0.11%) (Fig. S5b). We confirmed the multidirectional differentiation capacity of SuSCs through osteogenic, adipogenic, and chondrogenic induction assays (Fig. S5c, d, e).
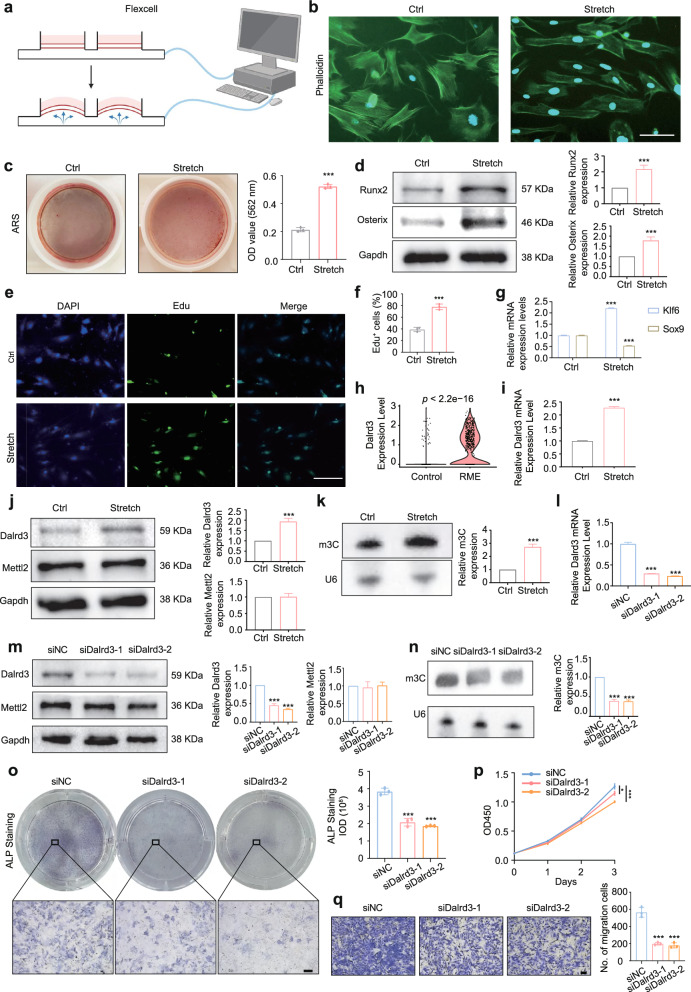
We then employed the Flexcell system to simulate cyclic stretch conditions in vitro (Fig. 5a). Based on parameters from previous studies [8, 21–24], we tested various frequencies and stretch magnitude to identify optimal mechanical conditions. The osteogenic differentiation of SuSCs was assessed by measuring the expression levels of Osx and Runx2 mRNA. Results indicated that frequencies above 0.5 Hz and stretch magnitude exceeding 5% led to a decline in osteogenic differentiation, likely due to cell death from excessive force. The optimal osteogenic differentiation was achieved with a 0.025 Hz sinusoidal curve at 4% elongation (Fig. S5f, g, h). After applying cyclic tension, cytoskeletal staining revealed a transformation in cell morphology from irregular triangles to elongated spindles, with cells aligning in a specific direction (Fig. 5b). Additionally, there was an increase in Alizarin Red-positive calcium nodules post-stretch (Fig. 5c). Analysis showed an upregulation of osteogenic proteins (Runx2, Osterix) and enhanced cell proliferation as indicated by EdU staining (Fig. 5d, f). Moreover, qRT-PCR demonstrated increased expression of the stemness-related gene Klf6 and decreased expression of Sox9, linked to chondrogenic differentiation (Fig. 5g). These findings align with scRNA-seq results, confirming our establishment of an in vitro model for osteogenic differentiation of SuSCs under mechanical tension.
Fig. 5.
Mechanoresponsive Dalrd3 promotes osteogenic differentiation of SuSCs. a Schematic illustration of cyclic stretch conditions applied to SuSCs. b Representative images of SuSCs stained for F‐actin (green) and nuclei (blue) under mechanical stretch (Control: 0 Hz, 0% elongation; Stretch: 0.025 Hz, 4% elongation). Scale bar: 100 μm. c Representative images and quantitative analysis of Alizarin Red S (ARS) staining in SuSCs following an additional 4-day osteogenic induction after 3 days of mechanical stretching. (n = 3) d Western blot analysis of Runx2 and Osterix expression in SuSCs from control (ctrl) and stretched (stretch) groups. (The figure presented is the cropped gel. Full-length gels are presented in Supplementary Fig. 6. Both the target protein and the internal control were derived from the same batch of samples). (n = 3) e–f Representative images and quantitative analysis of EdU immunofluorescence staining in SuSCs from control and stretched groups, indicating proliferative capacity. (n = 3) Scale bar: 100 μm. g Relative mRNA expression levels of stemness-related gene Klf6 and chondrogenic differentiation marker Sox9 in control and stretched SuSCs. (n = 3) h Violin plots displaying Dalrd3 gene expression in SuSCs from control and RME groups as determined by scRNA-seq. i Relative mRNA expression level of Dalrd3 in control and stretched SuSCs. (n = 3) j Western blot analysis of Dalrd3 and Mettl2 expression in SuSCs from control and stretched groups. (the figure presented is the cropped gel. Full-length gels are presented in Supplementary Fig. 7. Both the target protein and the internal control were derived from the same batch of samples). (n = 3) k Northwestern blotting analysis of m3 C modification in tRNAs from control and stretched SuSCs. (the figure presented is the cropped gel. Full-length gels are presented in Supplementary Fig. 8). (n = 3) l Validation of Dalrd3 knockdown effects in SuSCs via qRT-PCR. m Western blot analysis of Dalrd3 and Mettl2 expression in SuSCs following siNC and siDalrd3 treatment. (the figure presented is the cropped gel. Full-length gels are presented in Supplementary Fig. 9. Both the target protein and the internal control were derived from the same batch of samples). (n = 3) n Northwestern blotting of m3 C modification in tRNAs from siNC and siDalrd3-treated SuSCs. (the figure presented is the cropped gel. Full-length gels are presented in Supplementary Fig. 10). (n = 3) o Representative images and quantitative analysis of ALP staining in siNC and siDalrd3-treated SuSCs. (n = 3) Scale bar: 200 μm. p-q Dalrd3 knockdown reduces SuSCs’ proliferation (p) and migration (q) abilities, as demonstrated by cell proliferation and migration assays. (n = 3) Scale bar: 100 μm. *P < 0.05, ***P < 0.001. Student’s t test comparisons to control group (Ctrl, control, and siNC) was used
Next, we examined the expression of Dalrd3 in SuSCs under mechanical tension. Consistent with scRNA-seq results (Fig. 5h), qRT-PCR and western blotting showed a significant increase in Dalrd3 expression following mechanical stretch (Fig. 5i, j). Given that Mettl2 and Dalrd3 form complexes that mediate arginine tRNA m3 C modification, we assessed the effects of mechanical stretch on Mettl2 and tRNA m3 C modification. While Mettl2 levels remained unchanged (Fig. 5j), tRNA m3 C modification significantly increased (Fig. 5k). This suggests that Dalrd3 upregulation in SuSCs may regulate tRNA m3 C modification levels under mechanical stretch. We then inhibited Dalrd3 using siRNAs, and validated through qRT-PCR and western blotting (Fig. 5l, m). Knockdown of Dalrd3 did not affect Mettl2 levels but reduced tRNA m3 C modification levels (Fig. 5m, n). Functional experiments revealed that Dalrd3 knockdown impaired osteogenic differentiation and diminished proliferation and migration abilities of SuSCs (Fig. 5o, p). These results indicate that Dalrd3 plays a crucial role in regulating the osteogenic differentiation of SuSCs, potentially through its influence on tRNA m3 C modification.
Dalrd3-mediated tRNA m3 C modification regulates Id3 mRNA translation in SuSCs
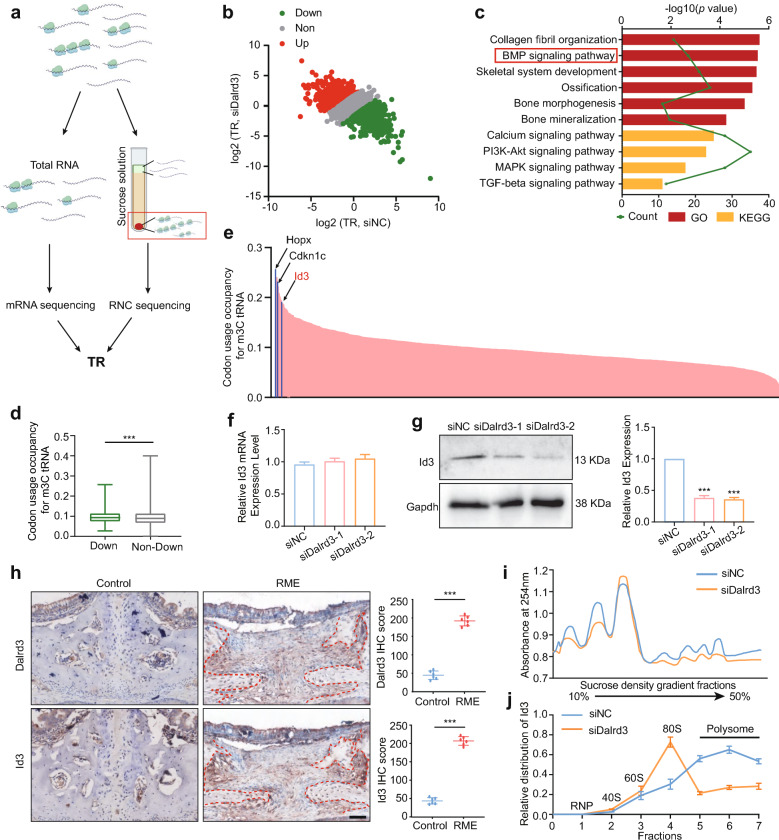
Given the critical role of tRNA modifications in protein translation, we hypothesize that mechanical tension elevates Dalrd3 levels in SuSCs, which subsequently impacts osteogenic differentiation at the translational level. To investigate how Dalrd3 regulates this process, we conducted RNC-seq on SuSCs treated with siDalrd3 and siNC (Fig. 6a). GO and KEGG analyses showed that mRNAs with reduced TRs (Fig. 6b) were significantly enriched in the BMP signaling pathway (Fig. 6c).
Fig. 6.
Regulation of Id3 translation via Dalrd3-mediated tRNA m3 C modification. a Schematic representation of RNC-seq methodology. b Scatterplot of TRs in siNC and siDalrd3 SuSCs, calculated as the ratio of ribosome-bound transcripts to input RNA-seq signals. c GO and KEGG enrichment analysis of genes with decreased TRs following Dalrd3 knockdown. d Comparison of m3 C tRNA-decoded codons in mRNAs with downregulated TRs (down) versus non-downregulated TRs (non-down) in Dalrd3-depleted SuSCs. e Bar graph displaying codon usage scores for m3 C tRNA-decoded codons in mRNAs with downregulated TRs; top three genes associated with the BMP signaling pathway are highlighted. f qRT-PCR analysis showing that Id3 mRNA levels remain unchanged upon Dalrd3 knockdown. (n = 3) g Western blot analysis demonstrating a significant reduction in Id3 protein levels due to Dalrd3 knockdown. (the figure presented is the cropped gel. Full-length gels are presented in Supplementary Fig. 11). (n = 3) h Representative immunohistochemistry (IHC) staining and quantification of Dalrd3 and Id3 in the midpalatal suture of control and RME mice. (n = 6) Scale bar:50 μm. i Polysome profile assay indicating an overall reduction in translation efficiency in SuSCs following Dalrd3 knockdown. j qPCR analysis of Id3 mRNA distribution across polysome gradient fractions, comparing siNC and siDalrd3 SuSCs. ***P < 0.001. Student’s t test was used
To explore the relationship between mRNA translation and Arg-tRNA m3 C modification, we calculated codon usage for Arg-tRNAs in differentially translated mRNAs. Our findings showed that mRNAs with reduced translation rates contained a significantly higher number of codons recognized by m3 C-Arg-tRNAs (Fig. 6d). We then calculated the codon usage score of m3 C-Arg-tRNA in these genes with decreased TRs and found genes related to BMP signaling pathway had high codon usage scores (Fig. 6e), for example, HOP Homeobox (Hopx), Cyclin Dependent Kinase Inhibitor 1 C (Cdkn1c) and Id3. Notably, Id3 is a critical downstream target of the BMP signaling pathway [31]. suggesting its importance as a target of Dalrd3.
To explore whether Dalrd3 regulates Id3 protein expression at the translational level, we conducted qRT-PCR analysis, which showed that Dalrd3 inhibition in SuSCs did not affect Id3 transcription (Fig. 6f). However, western blotting revealed a significant decrease in Id3 protein levels (Fig. 6g). Further validation through IHC showed that RME group exhibited significantly higher Dalrd3 and Id3 expression levels compared to the control group (Fig. 6h). To examine whether Dalrd3 knockdown affects global translation, polysome profiling assays demonstrated reduced translation efficiency in siDalrd3 SuSCs (Fig. 6i). Subsequent qRT-PCR analysis of different sucrose gradient fractions showed that in siDalrd3 SuSCs, Id3 mRNA was primarily found in monosomes and light polysomes, while in siNC SuSCs, it was predominantly located in polysomes, suggesting a decrease in translating Id3 mRNA following Dalrd3 knockdown (Fig. 6j). These findings suggest that Dalrd3 plays a role in regulating translation.
To confirm the role of Id3 in Dalrd3 function in SuSCs, we conducted a rescue assay by overexpressing Id3 in Dalrd3-depleted SuSCs. (Fig. 7a). The results showed that Id3 overexpression rescued osteogenic differentiation, proliferation, and migration abilities in Dalrd3-depleted SuSCs (Fig. 7b, c d e).
Fig. 7.
Id3 restoration of osteogenic differentiation in Dalrd3-depleted SuSCs. a Western blot analysis using indicated antibodies in SuSCs with Dalrd3 knockdown, with or without Id3 overexpression. (the figure presented is the cropped gel. Full-length gels are presented in Supplementary Fig. 12). b-c Representative images (b) and quantitative analysis (c) of ALP staining in Dalrd3 knockdown SuSCs, with or without Id3 overexpression. (n = 3) Scale bar: 100 μm. d-e Restoration of proliferative capacity (d) and migratory ability (e) in Dalrd3-depleted SuSCs via Id3 overexpression, as shown in cell proliferation and migration assays. (n = 3) Scale bar: 100 μm. f Proposed working model illustrating how Dalrd3-mediated tRNA m3 C modification regulates osteogenic differentiation of SuSCs under mechanical stretch. **P < 0.01, ***P < 0.001. One-way ANOVA with Dunnett’s multiple comparisons to the siNC group was used
Overall, these findings indicate that in the midpalatal suture, SuSCs undergo osteogenic differentiation upon mechanical tension, and the expression level of Dalrd3 is increased, which upregulates the BMP signaling pathway at the translation level. Mechanically, Id3 is identified as a critical downstream target of Dalrd3, supporting the conclusion that Dalrd3-mediated tRNA m3 C modification promotes osteogenic differentiation in SuSCs through regulating Id3 (Fig. 7f).
Discussion
Bone suture growth, a special form of intramembranous osteogenesis, represents a distinctive growth pattern in craniomaxillofacial bones. Bone sutures respond to mechanical tension by stimulating bone growth during stretching, serving as the biological basis for orthodontic orthopedics. It is widely accepted that SuSCs can differentiate into osteoblast precursors, eventually maturing into osteoblasts upon mechanical stimulation [3]. However, the mechanisms of how mechanical forces stimulate suture and bone remodeling are not yet fully understood, posing challenges for effective expansion treatment. Despite numerous attempts to enhance bone remodeling after expansion, no approach has yet confirmed changes in the differentiation trajectory of SuSCs at the in vivo single-cell level, nor clarified the regulatory mechanisms governing epigenetic modifications and protein translation in SuSCs post-expansion. In this study, we aimed to address these gaps using scRNA-seq and RNC-seq, along with other advanced detection techniques. Our findings highlight two key insights: first, while SuSCs typically differentiate into chondroblasts, they shift toward osteogenic differentiation following expansion in midpalatal suture. Second, we identified a significant increase in the expression of the epigenetic factor Dalrd3 and tRNA m3 C modification levels in SuSCs, which subsequently led to enhanced translation of Id3 mRNA and activation of the BMP signaling pathway to promote osteogenic differentiation of SuSCs.
In craniomaxillofacial sutures, SuSCs are heterogeneous mixtures of several stem cell lineages that express markers like Prx1/Prrx1 [13], Ctsk [32], Gli1 [18], or Axin2 [33]. Mesenchymal stem cells (MSCs) express markers such as Lepr, Ly6a/Sca-1, Cd44, Lgr5, or Cxcl12 [34]. Their origins are complex and vary according to developmental stage and tissue specificity. Previous studies on bone regeneration after suture extension have primarily focused on Gli+ and Prx1/Prrx1+ SuSCs [11, 12, 14]. However, it is not a single stem cell population drives bone growth in these sutures, and these stem cell populations often exhibit subcellular co-expression and co-localization. Jing et al. demonstrated that bone remodeling continues even after the Gli1+ SuSCs are knocked out upon expansion [14]. In fact, scRNA-seq is exceptionally well-suited for exploring the heterogeneity of SuSCs. In this study, SuSCs exhibited signaling pathways regulating pluripotency of stem cells and responding to mechanical stimulus. Furthermore, we found that SuSCs expressed a variety of stem cell markers, with Ctsk and Prrx1 being the most prevalent, and individual SuSCs were capable of expressing multiple markers simultaneously. These findings support the diversity of stem cell markers and reveal both homogeneity and heterogeneity among the various stem cell populations in craniomaxillofacial sutures.
Mechanotransduction is a tightly regulated process by which organisms detect mechanical stimuli and convert them into downstream biochemical responses. In cellular behaviors, mechanical signals influence behaviors such as cell migration, differentiation, proliferation, apoptosis, and overall functional regulation [35]. In this study, we observed that mechanical tension induced mesenchymal cells to migrate into the suture. Additionally, SuSCs displayed a shift toward osteogenic rather than chondrogenic differentiation, along with enhanced cell proliferation in response to mechanical tension. At the subcellular and molecular levels, forces are transmitted from extracellular matrix to nucleus through various mechanisms, including protein conformational changes [36], integrin-adhesion [37], cell–cell adhesion [38], cytoskeleton [39], nuclear mechanotransduction [40] and ion channels [41]. These forces also directly impact epigenetic modifications and chromosomal states, leading to long-term changes in genomic activity [42].
Research has primarily focused on transcriptional regulation of osteogenic differentiation in response to mechanical expansion [43]. However, recent studies have highlighted the significant role of translation regulation in bone development. For instance, mTORC1, a dominating regulator of translation, and ribosome protein play critical roles in bone development [44, 45]. Stem cells are characterized by low global translation rates, and the regulation of translation is essential for their transition to differentiation [28, 46]. This process is complex and requires precise regulation. Factors influencing translation in stem cells include the characteristics and abundance of tRNAs [47]. For example, the abundance of m7G-modified tRNAs regulates bone development through selective mRNA translation [48]. Nonetheless, the role of tRNAs in mediating osteogenic differentiation under mechanical expansion remains uncertain. In this study, we found that the translation levels of SuSCs increased under mechanical tension, alongside elevated levels of Dalrd3 and tRNA m3 C modification. While the knockdown of Dalrd3 resulted in decreased tRNA m3 C modification and translation levels, leading to a reduced osteogenic differentiation ability of SuSCs. The limitation of this study lies in the need for further in vivo validation of the role of Dalrd3 in suture distraction osteogenesis using transgenic mice. DALRD3 mutations cause loss of tRNA-Arg m3 C modification, leading to microcephalia [30]. However, whether Dalrd3-tRNA-m3 C affects whole-body bone development requires further investigation.
In this study, knockdown of Dalrd3 resulted in reduced translation level of Id3. The ID family of basic helix-loop-helix (bHLH) proteins are early, direct targets of the BMP pathway. Moreover, ID3 is a critical effector of BMP-induced osteoblastogenesis [49]. BMP signaling is essential for regulating various stem cell functions in craniofacial development [50]. Previous studies have shown that the activation of the BMP pathway is primarily driven by increased BMP ligand expression, enhanced receptor activity, and the inhibition of negative regulators [51]. However, in cranial neural crest cells, BMP signaling activation is essential for glycolytic lactate production, which leads to epigenetic histone lactylation and results in craniofacial anomalies [52]. Interestingly, our findings reveal a novel epigenetic mechanism regulating BMP signaling pathway. Mechanical tension increases the level of tRNA m3 C modification, subsequently enhances mRNA translation for genes with a high proportion of codons decoded by m3 C-tRNA, which elevates Id3 expression and enhances BMP signaling at the translational level.
Conclusions
Our study uncovered a new mechanism through which mechanical tension regulates cell fate. SuSCs respond to mechanical tension by increasing Dalrd3 expression, which elevates tRNA m3 C modification, and then enhances Id3 expression level and BMP signaling pathway in translation levels. This study advances the understanding of craniomaxillofacial bone development and provides valuable insights for treating suture expansion in craniofacial deformities.
Supplementary Information
Acknowledgements
The authors declare that they have not use AI-generated work in this manuscript.
Abbreviations
- SuSCs
Bone suture stem cells
- RME
Rapid maxillary expansion
- scRNA-seq
Single-cell RNA sequencing
- Dalrd3
DALR anticodon binding domain containing 3
- m3 C
3-Methylcytosine
- Id3
Inhibitor of DNA binding 3
- BMP
Bone morphogenetic protein
- ROIs
Regions of interest
- BMD
Bone mineral density
- BV/TV
Bone volume fraction
- UMAP
Uniform manifold approximation and projection
- GO
Gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- GSVA
Gene Set Variation Analysis
- DEGs
Differentially expressed genes
- FDR
False discovery rate
- RNC-seq
Ribosome nascent-chain complex-bound mRNA sequencing
- TRs
Translation ratios
- IHC
Immunohistochemistry
- ALP
Alkaline phosphatase
- IOD
Integrated optical density
- ARS
Alizarin red staining
- qRT-PCR
Quantitative real‐time polymerase chain reaction
Author contributions
JC and YW performed most part of the in vivo and in vitro experiments. JC designed the study, analyzed all the sequencing data and wrote the manuscript. CZ and GL made important contributions to animal experiments. ZF and YC supervised the study. All coauthors have reviewed and approved this version of the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (82403184), GuangDong Basic and Applied Basic Research Foundation (2023 A1515110475), the Natural Science Foundation of Guangdong Province, China (2024 A1515012316), and the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (24qnpy201).
Availability of data and materials
The datasets generated and analysed during the current study are available in the Genome Sequence Archive repository (CRA020234). Shared URL: https://ngdc.cncb.ac.cn/gsa/search?searchTerm=CRA020234
Declarations
Ethics approval and consent to participate
For animal experiments, the study entitled “The mechanism of Dalrd3 regulating the osteogenic differentiation of bone suture stem cells during the expansion of the midpalatal suture in C57BL/6 mice” was approved by the Laboratory Animal Center of Sun Yat-Sen University approved the experiments conducted (Date:07.12.2024, No. SYSU-IACUC-2024–002015).
Consent for publication
No applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Chen and Yiwei Zhao have contributed equally to this work.
Contributor Information
Zhicai Feng, Email: fengzhc@mail.sysu.edu.cn.
Yang Cao, Email: caoyang@mail.sysu.edu.cn.
References
- 1.Holmes G, Gonzalez-Reiche AS, Lu N, Zhou X, Rivera J, Kriti D, et al. Integrated transcriptome and network analysis reveals spatiotemporal dynamics of calvarial suturogenesis. Cell Rep. 2020;32(1): 107871. 10.1016/j.celrep.2020.107871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalmar CL, Wes AM, Mazzaferro DM, Bartlett SP, Taylor JA. Forces exerted in craniofacial distraction osteogenesis. J Craniofac Surg. 2022;33(1):187–91. 10.1097/SCS.0000000000008283. [DOI] [PubMed] [Google Scholar]
- 3.Liang W, Zhao E, Li G, Bi H, Zhao Z. Suture cells in a mechanical stretching niche: critical contributors to trans-sutural distraction osteogenesis. Calcif Tissue Int. 2022;110(3):285–93. 10.1007/s00223-021-00927-z. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Barriales M, Lafuente-Ibáñez de Mendoza I, Alonso-Fernández Pacheco JJ, Aguirre-Urizar JM. Rapid maxillary expansion in paediatric obstructive sleep apnoea. Lancet Respir Med. 2023;11(5):e45. 10.1016/S2213-2600(23)00099-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Wang X, Jin A, Wang M, Wang Z, Huang X, et al. Reducing relapse and accelerating osteogenesis in rapid maxillary expansion using an injectable mesoporous bioactive glass/fibrin glue composite hydrogel. Bioact Mater. 2022;18:507–25. 10.1016/j.bioactmat.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Zhang Z, Gu X, Jin Y, Feng C, Yang S, et al. MicroRNA-21 affects mechanical force-induced midpalatal suture remodelling. Cell Prolif. 2020;53(1): e12697. 10.1111/cpr.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koehne T, Kahl-Nieke B, Amling M, Korbmacher-Steiner H. Inhibition of bone resorption by bisphosphonates interferes with orthodontically induced midpalatal suture expansion in mice. Clin Oral Investig. 2018;22(6):2345–51. 10.1007/s00784-018-2335-z. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Zhao F, Liang C, Hu L, Li D, Zhang Y, et al. Silencing of miR-138-5p sensitizes bone anabolic action to mechanical stimuli. Theranostics. 2020;10(26):12263–78. 10.7150/thno.53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Xu T, Zhang L, Li Y, Yan T, Yu G, et al. Midpalatal suture: single-cell RNA-seq reveals intramembrane ossification and piezo2 chondrogenic mesenchymal cell involvement. Cells. 2022;11(22):3585. 10.3390/cells11223585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou B, Fukai N, Olsen BR. Mechanical force-induced midpalatal suture remodeling in mice. Bone. 2007;40(6):1483–93. 10.1016/j.bone.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Li Z, Liu P, Wu M, Liu AQ, Hu C, et al. Gli1+ cells residing in bone sutures respond to mechanical force via IP(3)R to mediate osteogenesis. Stem Cells Int. 2021;2021:8138374. 10.1155/2021/8138374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldawood ZA, Mancinelli L, Geng X, Yeh SA, Di Carlo R, Leite TC, et al. Expansion of the sagittal suture induces proliferation of skeletal stem cells and sustains endogenous calvarial bone regeneration. Proc Natl Acad Sci. 2023;120(16):e2120826120. 10.1073/pnas.2120826120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilk K, Yeh SA, Mortensen LJ, Ghaffarigarakani S, Lombardo CM, Bassir SH, et al. Postnatal calvarial skeletal stem cells expressing PRX1 reside exclusively in the calvarial sutures and are required for bone regeneration. Stem Cell Reports. 2017;8(4):933–46. 10.1016/j.stemcr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing D, Chen Z, Men Y, Yi Y, Wang Y, Wang J, et al. Response of Gli1+ suture stem cells to mechanical force upon suture expansion. J Bone Miner Res. 2022;37(7):1307–20. 10.1002/jbmr.4561. [DOI] [PubMed] [Google Scholar]
- 15.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86. 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 16.Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13(1):33–42. 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Wu T, Fan F, Liu Y, Wu L, Junkin M, et al. A portable and cost-effective microfluidic system for massively parallel single-cell transcriptome profiling. bioRxiv.2019:818450.
- 18.Zhao H, Feng J, Ho TV, Grimes W, Urata M, Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015;17(4):386–96. 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Zhao J, Wang J, Sun L, Xu H, Sun W, et al. ROCK-TAZ signaling axis regulates mechanical tension-induced osteogenic differentiation of rat cranial sagittal suture mesenchymal stem cells. J Cell Physiol. 2020;235(9):5972–84. 10.1002/jcp.29522. [DOI] [PubMed] [Google Scholar]
- 20.Lin W, Li Q, Zhang D, Zhang X, Qi X, Wang Q, et al. Mapping the immune microenvironment for mandibular alveolar bone homeostasis at single-cell resolution. Bone Res. 2021;9(1):17. 10.1038/s41413-021-00141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong L, Song Y, Zhang Y, Zhao W, Wang C, Lin H, et al. Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J Cell Physiol. 2021;236(9):6376–90. 10.1002/jcp.30312. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Cai J, Zeng Z, Gao X, Shao X, Ding Y, et al. The interactions between mTOR and NF-κB: A novel mechanism mediating mechanical stretch-stimulated osteoblast differentiation. J Cell Physiol. 2020. 10.1002/jcp.30184. [DOI] [PubMed] [Google Scholar]
- 23.He YB, Liu SY, Deng SY, Kuang LP, Xu SY, Li Z, et al. Mechanical stretch promotes the osteogenic differentiation of bone mesenchymal stem cells induced by erythropoietin. Stem Cells Int. 2019;2019:1839627. 10.1155/2019/1839627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Liu Y, Ding W, Shi J, Li S, Liu Y, et al. Mechanical stretch-induced osteogenic differentiation of human jaw bone marrow mesenchymal stem cells (hJBMMSCs) via inhibition of the NF-κB pathway. Cell Death Dis. 2018;9(2):207. 10.1038/s41419-018-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Li K, Chen J, Wang X, Ling R, Cheng M, et al. Aberrant translation regulated by METTL1/WDR4-mediated tRNA N7-methylguanosine modification drives head and neck squamous cell carcinoma progression. Cancer Commun (Lond). 2022;42(3):223–44. 10.1002/cac2.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, Cui Y, Jin J, Guo J, Wang G, Yin X, et al. Translating mRNAs strongly correlate to proteins in a multivariate manner and their translation ratios are phenotype specific. Nucleic Acids Res. 2013;41(9):4743–54. 10.1093/nar/gkt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han C, Sun L, Pan Q, Sun Y, Wang W, Chen Y. Polysome profiling followed by quantitative PCR for identifying potential micropeptide encoding long non-coding RNAs in suspension cell lines. STAR Protoc. 2022;3(1): 101037. 10.1016/j.xpro.2021.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saba JA, Liakath-Ali K, Green R, Watt FM. Translational control of stem cell function. Nat Rev Mol Cell Biol. 2021;22(10):671–90. 10.1038/s41580-021-00386-2. [DOI] [PubMed] [Google Scholar]
- 29.Lin S, Liu Q, Lelyveld VS, Choe J, Szostak JW, Gregory RI. Mettl1/Wdr4-mediated m7G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol Cell. 2018;71(2):244-55.e5. 10.1016/j.molcel.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lentini JM, Alsaif HS, Faqeih E, Alkuraya FS, Fu D. DALRD3 encodes a protein mutated in epileptic encephalopathy that targets arginine tRNAs for 3-methylcytosine modification. Nat Commun. 2020;11(1):2510. 10.1038/s41467-020-16321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergmann S, Penfold CA, Slatery E, Siriwardena D, Drummer C, Clark S, et al. Spatial profiling of early primate gastrulation in utero. Nature. 2022;609(7925):136–43. 10.1038/s41586-022-04953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562(7725):133–9. 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon S, Salhotra A, Shailendra S, Tevlin R, Ransom RC, Januszyk M, et al. Skeletal stem and progenitor cells maintain cranial suture patency and prevent craniosynostosis. Nat Commun. 2021;12(1):4640. 10.1038/s41467-021-24801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen F, Shi Y. Recent advances in single-cell view of mesenchymal stem cell in osteogenesis. Front Cell Dev Biol. 2021;9: 809918. 10.3389/fcell.2021.809918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao R, Tian H, Tian Y, Fu X. A hierarchical mechanotransduction system: from macro to micro. Adv Sci (Weinh). 2024;11(11): e2302327. 10.1002/advs.202302327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sloas DC, Tran JC, Marzilli AM, Ngo JT. Tension-tuned receptors for synthetic mechanotransduction and intercellular force detection. Nat Biotechnol. 2023;41(9):1287–95. 10.1038/s41587-022-01638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saraswathibhatla A, Indana D, Chaudhuri O. Cell-extracellular matrix mechanotransduction in 3D. Nat Rev Mol Cell Biol. 2023;24(7):495–516. 10.1038/s41580-023-00583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troyanovsky SM. Adherens junction: the ensemble of specialized cadherin clusters. Trends Cell Biol. 2023;33(5):374–87. 10.1016/j.tcb.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehidi A, Kage F, Karatas Z, Cercy M, Schaks M, Polesskaya A, et al. Forces generated by lamellipodial actin filament elongation regulate the WAVE complex during cell migration. Nat Cell Biol. 2021;23(11):1148–62. 10.1038/s41556-021-00786-8. [DOI] [PubMed] [Google Scholar]
- 40.Kalukula Y, Stephens AD, Lammerding J, Gabriele S. Mechanics and functional consequences of nuclear deformations. Nat Rev Mol Cell Biol. 2022;23(9):583–602. 10.1038/s41580-022-00480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kefauver JM, Ward AB, Patapoutian A. Discoveries in structure and physiology of mechanically activated ion channels. Nature. 2020;587(7835):567–76. 10.1038/s41586-020-2933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater. 2013;12(12):1154–62. 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu S, Chen W, Masson A, Li YP. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024;10(1):71. 10.1038/s41421-024-00689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Long F. mTOR signaling in skeletal development and disease. Bone Res. 2018;6:1. 10.1038/s41413-017-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiu GC, Kerr CH, Forester CM, Krishnarao PS, Rosenblatt HD, Raj N, et al. A p53-dependent translational program directs tissue-selective phenotypes in a model of ribosomopathies. Dev Cell. 2021;56(14):2089-102.e11. 10.1016/j.devcel.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Signer RA, Magee JA, Salic A, Morrison SJ. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature. 2014;509(7498):49–54. 10.1038/nature13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzzi N, Cieśla M, Ngoc PCT, Lang S, Arora S, Dimitriou M, et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell. 2018;173(5):1204-16.e26. 10.1016/j.cell.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Jiang S, Lei K, Han H, Chen Y, Lin W, et al. Metabolic rewiring during bone development underlies tRNA m7G-associated primordial dwarfism. J Clin Invest. 2024;134(20): e177220. 10.1172/JCI177220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda Y, Tsuji K, Nifuji A, Noda M. Inhibitory helix-loop-helix transcription factors Id1/Id3 promote bone formation in vivo. J Cell Biochem. 2004;93(2):337–44. 10.1002/jcb.20154. [DOI] [PubMed] [Google Scholar]
- 50.Graf D, Malik Z, Hayano S, Mishina Y. Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine Growth Factor Rev. 2016;27:129–39. 10.1016/j.cytogfr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowery JW, Rosen V. The BMP Pathway and Its Inhibitors in the Skeleton. Physiol Rev. 2018;98(4):2431–52. 10.1152/physrev.00028.2017. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Zhu L, Pan H, Ueharu H, Toda M, Yang Q, et al. A BMP-controlled metabolic/epigenetic signaling cascade directs midfacial morphogenesis. J Clin Invest. 2024;134(8): e165787. 10.1172/JCI165787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available in the Genome Sequence Archive repository (CRA020234). Shared URL: https://ngdc.cncb.ac.cn/gsa/search?searchTerm=CRA020234