Abstract
Cognitive impairment (CI) is a significant and extraordinary complication of obstructive sleep apnea (OSA) patients. Programmed cell death (PCD) is an active and ordered process regulated by genes. A growing number of studies find that PCD is responsible for cognitive dysfunction and plays an important role in various neurological diseases, which involve apoptosis, necroptosis, pyroptosis, ferroptosis, and cell death associated with autophagy. However, the influence of PCD on OSA-CI remains unclear. We summarized the relevant studies that discussed the involvement of PCD in the CI of OSA and aimed to clarify the underlying mechanisms. Intermittent hypoxia (IH)-induced PCD had a critical effect on the mechanisms that produced the ultimate neurological deficit in OSA, and the PCD involved mainly included apoptosis, autophagy, ferroptosis, and pyroptosis. IH regulates PCD directly or through specific pathways, and drugs targeting related molecules have the potential to improve cognitive function. These findings enrich the pathogenesis of OSA-CI and provide new therapeutic insights.
Keywords: Obstructive sleep apnea, Cognitive impairment, Programmed cell death, Intermittent hypoxia
Introduction
Obstructive sleep apnea (OSA) is the most common adult sleep disorder [1]. It is characterized by intermittent hypoxia (IH), sleep fragmentation (SF), and hypercapnia due to repeated upper airway collapse during sleep [2]. The prevalence of OSA continues to rise, and it is estimated that there are nearly 1 billion adults aged 30–69 years old with OSA worldwide [3, 4]. A recent large cohort study suggested that better sleep consolidation and absence of OSA were associated with better cognition [5]. Cognitive impairment (CI) is an important complication of OSA, causing serious distress to the lives of patients [6].
Cell death is a basic physiological process in all living organisms and plays a crucial role in maintaining tissue homeostasis and preventing disease [7]. Abnormal cell death is involved in the occurrence and progression of diseases. Insufficient cell death can lead to cancer and persistent infection, while excessive cell death is thought to be associated with degenerative and inflammatory diseases [8]. Traditional classifications of cell death include programmed cell death (PCD) and non-PCD. PCD is a regulated form of active cell death, including apoptosis, pyroptosis, necroptosis, ferroptosis, autophagy, cuproptosis, etc. [7]. Abnormal activation of PCD signaling cascades can be observed in various neurological diseases in response to different stress and inflammatory stimuli, such as apoptosis, necroptosis, pyroptosis, ferroptosis, and cell death associated with autophagy [9, 10]. A growing number of studies show that PCD is an important mechanism of CI in OSA patients [11, 12]. However, the processes leading to PCD and the type of PCD remain unclear.
In this review, we summarized and explored the recent advances in the roles of PCD in OSA-CI to figure out the underlying molecular mechanisms (Fig. 1) and provide new therapeutic targets.
Fig. 1.
Regulatory pathways or evidence of programmed cell death in OSA-CI
Clinical and imaging evidence of OSA-CI
OSA-CI involves a wide range of cognitive function areas, including memory, attention/alertness, executive function, and visuospatial deficits [13, 14]. In addition, some OSA patients suffer from emotional disorders, and severe cases can lead to permanent brain damage [6]. There is substantial clinical and imaging evidence supporting CI in OSA patients. Magnetic resonance imaging (MRI) of OSA patients showed decreased gray matter (GM) concentration or atrophy in multiple brain regions, including cortex (frontal, temporal, parietal), subcortical areas (thalamus, hippocampus, amygdala, cingulate gyrus), and cerebellum [15–17]. At the same time, the integrity of white matter (WM) in OSA patients is changed. The corpus callosum, cingulate cortex, pyramidal tract, insular cortex, basal ganglia, and limbic regions are commonly affected areas, which are mainly involved in the regulation of emotion, autonomic nervous system, and cardiovascular system [18]. Recent evidence suggests that there are biphasic changes in GM and WM in OSA. The first pattern is characterized by neuroimaging markers of GM loss and damage, suggesting chronic cell loss and accelerated aging. The other pattern presents with GM hypertrophy and reduced WM diffusivity, which may be related to acute and reactive changes [17]. In recent years, diffusion tensor imaging (DTI) has been widely used to assess brain microstructural alterations in OSA. Decreased fractional anisotropy and elevated mean and radial diffusivity in the anterior corpus callosum have been associated with impairments in prospective memory and sustained attention among OSA patients [19]. The disruption of white matter integrity and connectivity in regions such as the cingulate gyrus, accessory cingulate gyrus, and amygdala in OSA patients was reported when used DTI to construct brain structural network data, these abnormalities might be the basis for the reduced efficiency of inter-regional communication and cognitive information processing [20]. Diffusion kurtosis imaging (DKI) is another method based on a non-Gaussian diffusion model. Previous studies have reported an increase in brain mean kurtosis (MK) values in patients with OSA, which may reflect acute brain tissue injury. The mechanism may include impaired cell membrane permeability and energy pump dysfunction caused by ischemia and hypoxia, as well as changes in the volume fraction of neural tissue (cytotoxic edema or swelling of axons and neurons) [21]. Other studies have detected decreased brain MK values in patients with more severe OSA or CI, and this difference may be due to neuronal and oligodendrocyte degeneration or even cell death caused by hypoxia or ischemia [22].
Basic mechanisms of OSA-CI
There are multiple mechanisms of CI in OSA. IH and SF were seen as important initiating factors. IH can increase the level of oxidative stress in vivo, especially in the brain, which is one of the important pathological mechanisms of OSA-CI [23, 24]. The increase of reactive oxygen species and reactive nitrogen species under oxidative stress can further promote intracellular signaling cascades, leading to the increase of pro-inflammatory gene expression, and the increase of inflammatory response further aggravates oxidative stress [25]. Similarly, oxidation-antioxidant imbalance in OSA may increase inflammation, leading to neuronal apoptosis and microglial cell activation [24]. IH-induced oxidative stress and inflammation can also cause CI by damaging the blood–brain barrier (BBB), especially changes in microvascular permeability. When BBB is damaged, blood-derived glial activation inducers infiltrate the brain and cause glial activation, then induce neuronal death. On the other hand, glial activation further triggers BBB dysfunction by releasing inflammatory mediators [26]. Besides, previous studies have shown that OSA has pathological protein deposition similar to neurodegenerative diseases [27–30]. Abnormal protein deposition promotes damage or even death of nerve cells in the brain, mediating cognitive changes [31].
In OSA patients, SF is independently associated with CI including decreased sustained attention, impaired reaction time, and visuospatial deficits [32]. SF in OSA patients is associated with the unique patterns of insufficient cerebral perfusion, the regions with reduced perfusion overlap with the main areas of the default mode network and the attention network, indicating impaired attention and executive functions [33]. In addition, Alzheimer’s disease biomarkers and complement proteins derived from neural cell exosomes are regarded as mediating effects between sleep fragmentation and CI in patients with OSA [34]. In the mouse model, SF exposure led to increased oxidative stress [35, 36], neuroinflammation, microglia activation, and enhanced BBB permeability, resulting in decreased cognitive function [37].
PCD of OSA-CI
Apoptosis
Apoptosis was the earliest identified form of PCD, which is characterized by membrane blebbing, decreased cell size, nuclear fragmentation, chromatin condensation, exposure of phosphatidylserine on the cell surface, and apoptotic body formation [7, 38]. Apoptosis can be initiated through either an intrinsic (mitochondrial pathway of apoptosis) or an extrinsic pathway (death receptor-mediated apoptosis) [38]. Intrinsic apoptosis can be activated by intracellular signals such as DNA damage, Ca2+ overload, and elevated levels of reactive oxygen species (ROS), while extrinsic apoptosis is induced by external ligands that bind to cell surface death receptors [39]. OSA-CI is closely related to apoptosis (Table 1).
Table 1.
Apoptosis in the cognitive impairment of OSA model
| Year | PMID | IH and SF model | Brain region/cell | Apoptosis Markers (methods) | Regulation mechanism | Behavior test |
|---|---|---|---|---|---|---|
| 2012 | 22932184 | SD rats: O2 level from 21 to 10% for 5 s every 90 s, 8 h/d, 4 weeks | Hippocampus/neuron | Apoptotic Index↑ (TUNEL) | Negatively correlated with thioredoxin | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
| 2015 | 26419512 | SD rats: O2 decreased to a nadir level of 6.5–7% in 25–30 s and sustained for 30–35 s, then rose to 21% and lasted about 50 s. 2 min/cycle, 8 h/d, 30 days | Prefrontal cortex and hippocampus/neuron | Apoptotic cells↑, cleaved caspase-3↑, Bax/Bcl2↑ (TUNEL and WB) | – | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
| 2015 | 25843188 | C57BL/6 J mice: IH consists of cycles of oxygen levels between 10 and 21% every 90 s, 40 cycles per hour, 8 h/d, 14 days | Hippocampus (CA1)/neuron | Percentage of apoptotic cells↑, Bcl2↓, cleaved caspase-3↑ (TUNEL and WB) | Endoplasmic reticulum stress↑ |
Open-field exploration: no significant difference Delay-dependent one-trial object recognition task: the percentage of preference for the novel object↓ Eight-arm maze: reference memory errors↑ |
| 2016 | 26996481 | C57/BL mice: O2 level was reduced from 21 to 8% in 120 s, held at 8% for 120 s, returned to 21% for 50 s, and held at 21% for 300 s, 8 h/d, 5 weeks | Hippocampal dentate gyrus region/neuron | Apoptotic Index↑ (TUNEL) | Wnt Signaling Pathway (GSK-3β activity↑ and β-catenin↓) | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
| 2019 | 31678553 |
C57BL/6 J mice, KKAy type 2 diabetes model mice: (O2 concentration decreased from 21 to 5% over 30 s and then rebounded to 21% and was maintained 90 s) 5–21% O2, 30 cycles per hour, 8 h/d, 4 weeks HT22 cell: 1.5% O2 for 30 s and 21% O2 for 90 s, maintained 8 h |
Hippocampus/neuron | Cleaved caspase-3↑, Bax/Bcl2↑ (WB and IHC) | HMGB1/TLR4 signaling pathway↑ | – |
| 2020 | 32209423 | C57BL/6 mice: O2 decreased from 21 to 9% for 1.5 min and then gradually returned to 21% within 1.5 min. 20 times/h, 8 h/d, 3 weeks | Hippocampus (CA1, CA3, dentate gyrus)/neuron | Cleaved Caspase-3/pro- caspase-3↑, cleaved PARP/pro- PARP↑, Bcl2/Bax↓, apoptotic cells↑ (WB and TUNEL) | Iron overload |
Morris water maze: escape latency↑, crossing number↓ Open field maze: time in open field↓, distance in center field↓ |
| 2020 | 32200526 | Wistar rats: O2 concentration dropped from 21 to 8% in 1 min and remained at 8% for 2 min, then it went from 8 to 21% in 1 min and stayed at 21% for 2 min, 8 h/d, 4 weeks | Hippocampus/neuron | Apoptotic cells↑, Bax↑, Bcl2↓, caspase-3↑ (TUNEL and WB) | Nrdp1↑ | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
| 2021 | 34471408 |
C57BL/6 J mice: concentration of O2 was maintained between 10 and 21%, cycling every 90 s for 8 h, 14 days PC12 cells: 8 episodic cycles of 21% O2 for 25 min and 0.1% O2 for 35 min |
Hippocampus/neuron |
Apoptotic cell ratio↑, cleaved caspase-3↑ in mitochondria: Bax↑, Bak↑, cytochrome c↓ in cytoplasm: Bax↓, Bak↓, cytochrome c↑ (flow cytometry analysis and WB) |
PERK-ATF4-CHOP pathway↑ |
Open-field exploration: no significant difference Delay-dependent one-trial object recognition task: the percentage of preference for the novel object↓ Morris water maze: escape latency↑, number of attempts required to find the platform↑ |
| 2022 | 35030992 | C57BL/6 mice: The O2 level was maintained at 7% for 15–20 s, recovered to 21% in 45–50 s, and sustained for 15–20 s. 20 cycles per hour, 8 h/d, 3 months | Hippocampal dentate gyrus region/neuroblast | Cleaved caspase-3↑ (IF) | Abnormal lipid droplet accumulation |
Three-chamber social test: social novelty cognition↓ Morris water maze: escape latency↑, crossing number↓, dwell time↓, distances in the target quadrant↓ Fear conditioning test: fear memory impairment |
| 2023 | 37553048 |
C57BL/6NJ mice: O2 ranged from 21.0% ± 0.5% to 9.0% ± 1.5% in 90 s every cycle, 7.5 h/d, 4 weeks PC12 cells: hypoxia (94%N2/1% O2/5%CO2) for 1 h and reoxygenation (74%N2/21%O2/5%CO2) for 30 min, a total of 6 cycles |
Hippocampus (CA1)/neuron | Apoptotic index↑, cleaved caspase-3↑, Bcl2/Bax↓ (TUNEL and WB) | CaSR↑ induced PERK-ATF4-CHOP pathway↑ | Eight-arm maze: working memory errors↑, reference memory errors↑, total errors↑ |
| 2023 | 36600080 |
C57BL/6 mice: O2 fluctuated from 9.0% ± 1.5% to 21% ± 0.5% in 90 s per cycle, 40 cycles/h, 7 h/d, 4 weeks PC12 cells: hypoxia (94%N2/1% O2/5%CO2) for 1 h and reoxygenation (74%N2/21%O2/5%CO2) for 30 min, a total of 6 cycles |
Hippocampus (CA1)/neuron | Apoptotic index↑, cleaved caspase-3↑, Bcl2/Bax↓ (TUNEL and WB) | CaSR-PKC-ERK1/2 pathway↑ | Eight-arm maze: working memory errors↑, reference memory errors↑, total errors↑ |
| 2023 | 37254290 | C57BL/6 mice: alternating room air and 10% O2 every 90 s, and the replacement was realized within 10–30 s, 8 h/d, 4 weeks. Control the arousal frequency at 30 times/h | Hippocampus/neuron | Apoptotic cells↑ (TUNEL) | Nrf2 knockout increases apoptosis |
Eight-arm maze: IH: working memory errors↑, reference memory errors↑, total errors↑ SF: reference memory errors↑, total errors↑ |
| 2023 | 37140776 |
SD rats: O2 fluctuated from 7.5 ± 0.5% to 21 ± 0.5%, 8 h/d, 4 weeks HT22 cell: 5% O2 for 30 min, and 21% O2 for 30 min, 5% CO2, 8 h in total |
Hippocampus/neuron | Apoptotic rate↑, Bax↑, Bcl2↓, cleaved caspase-3/pro-caspase-3↑ (WB and flow cytometry analysis) | TGF-β3↓-Nrf-2/KEAP1/HO-1 pathway↑ | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
| 2023 | 37268259 | HT22 cell: 0.1% O2 3 min and 21% O2 7 min for 6 cycles/h, 48 h | – | Bcl2/Bax↓, cleaved caspase-3/caspase-3↑, fluorescence intensity of Annexin V-FITC (WB and apoptosis kit) | Autophagy↓ | – |
| 2023 | 37137262 |
C57BL/6J mice: The O2 level was maintained at 10% for 2 min, and recovered to 21% and sustained for 2 min. the replacement was realized within 2 min, 8 h/d, 4 weeks HT22 cell: O2 level alternate between 1 and 21% within 400 s, maintained 12 h |
Hippocampus/neuron | Apoptotic cells and rate↑ (TUNEL, flow cytometry analysis) | – | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
| 2024 | 37977071 |
C57BL/6 mice: O2 levels within the cage were alternated between 6% for 30 s and 21% for 90 s, 8 h/d, 4 weeks HT22 cell: O2 in the incubator was alternated between 1% for 100 s and 21% for 100 s, maintained for 10 h |
Hippocampus/neuron | Percentage of apoptotic cells↑ (TUNEL, flow cytometry analysis, CCK8) | – | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
TUNEL TdT-mediated dUTP Nick-End Labeling, WB western blot, IF immunofluorescence; IHC immunocytochemistry
IH-induced neuronal apoptosis and CI seem to increase with the duration of hypoxia within a certain range. A novel object recognition (NOR) test was performed to evaluate the changes in the cognitive function of mice, significant reductions in the recognition index were observed that progressed over the first 45 days and stabilized thereafter [40]. In the IH rat model, it was found that neuronal apoptosis in the hippocampus and cortex was significantly increased, and the results of the water maze suggested that cognitive decline and damage were more severe after 4 weeks of IH treatment than after 2 weeks [41]. Si et al. [42] treated HT22 (mouse hippocampal neurons) cells with IH for 48 h and found that cell activity gradually decreased from 12 h of intervention.
It seems that most IH-induced neuronal apoptosis belongs to intrinsic apoptosis, which can be regulated by multiple upstream pathways, and occurs mostly in the hippocampus and cortex. IH-induced neuronal apoptosis is closely related to oxidative stress. IH-treated rats could detect increased neuronal apoptosis in the hippocampus, which is negatively correlated with the expression of antioxidant thioredoxin [43]. Transforming growth factor-β3 (TGF-β3) exerts a protective effect on hippocampal neurons by binding to the TGF-β type receptor I (TGF-βRI), this neuroprotective effect is mediated by activation of the nuclear transcription factor 2 (Nrf-2)/Kelch-like ECH associated protein 1 (KEAP1)/hemo oxygenase 1 (HO-1) pathway, which strengthens the antioxidant defense and supports neuronal survival [44].
Endoplasmic reticulum stress (ERS) signaling can promote apoptosis [45], and acts as the linkage between IH and neuronal cell apoptosis. Increased ERS was found in the hippocampus of IH-treated mice, inducing neuronal apoptosis by upregulation of C/EBP homologous proteins (CHOP) and caspase-12, oxidative stress, and mitochondrial dysfunction [46]. After IH treatment, Bcl2 associated X protein (Bax) and Bcl2 antagonist 1 (Bak) were accumulated in mitochondria, which induced the release of cytochrome c and initiated cell apoptosis in the hippocampus of mice, and this process was regulated by the protein kinase-like endoplasmic reticulum kinase (PERK)- activating transcription factor 4 (ATF4)-CHOP pathway [47]. Another study also focused on ERS and apoptosis, they found calcium sensitive receptor (CaSR) increased, and mediated neuronal apoptosis through the PERK-ATF4-CHOP pathway under IH condition [48]. On the other hand, CaSR accelerated apoptosis and synaptic plasticity deficit by upregulating protein kinase C (PKC) and extracellular signal-regulated kinases 1/2 (ERK1/2) [49].
Specific ubiquitin ligases, glycogen synthase kinases, and inflammatory pathways, as well as autophagy and iron overload, have been reported to be involved in IH-induced neuronal apoptosis. IH could promote the expression of neuregulin receptor degradation protein-1 (NRDP1), which leads to neuronal apoptosis [50]. Similar neuronal apoptosis was also found in the hippocampus of the IH-exposed mice, and this might be regulated by increased activity of glycogen synthase kinase-3β (GSK-3β) and decreased β-catenin expression [51]. IH may induce apoptosis in hippocampal neurons by brain iron overload [52]. A recent study has shown that IH could promote neuronal apoptosis and oxidative stress by reducing levels of autophagy [42]. Besides, IH could promote neuronal apoptosis in normal mice and diabetes mice models, the latter had higher apoptosis levels and could be modulated by high mobility group box-1 protein (HMGB1)/toll-like receptor 4 (TLR4) signal [53]. IH-induced apoptosis of neuroblasts has also received attention apart from neurons. Li et al. detected abnormal lipid droplet accumulation in the hippocampus of IH-treated mice, and this change promoted neuroblast apoptosis [54].
In addition, it should be noted that SF is also an important pathogenic mechanism of OSA-CI [55], but there is little literature focusing on the effect of SF on neuronal apoptosis. A recent study has found that IH and SF treatment in mice can regulate hippocampal neuronal apoptosis through Nrf2 [35].
Autophagy
Autophagy encompasses different pathways that route cytoplasmic material to lysosomes for degradation, including 3 main types: macroautophagy, microautophagy, and chaperone-mediated autophagy [56]. It helps maintain cell homeostasis and recycle nutrients while removing toxic cellular components. However, under abnormal conditions (such as nutrient deprivation, oxidative stress, or exposure to cytotoxic agents), dysregulated autophagy can lead to cell death [7]. The normal function of the nervous system is thought to be highly dependent on autophagy because post-mitotic neurons are unable to dilute abnormal protein and organelle accumulation through cell division [56].
At present, studies on autophagy in OSA patients are pretty insufficient, and there is a lack of relevant clinical evidence to clarify the relationship between cognitive function and the level of autophagy in OSA patients. Abnormal expression of autophagy markers was observed in OSA patients. Previous studies have reported that Beclin-1 levels in the peripheral circulation of OSA patients increased with disease severity [57]. Peripheral blood cells of OSA patients showed impaired autophagy activity (decreased expression of LC3B/ATG5/BECN1/ULK1 and increased accumulation of p62) and increased DNA methylation in the promoter region of the LC3B gene, meanwhile corresponding in vitro studies showed similar autophagy trends, suggesting that IH-induced epigenetic regulation of autophagy damage [58].
Different IH conditions result in different autophagy patterns in hippocampal neurons, the duration, mode, and object of hypoxia all have effects (Table 2). Song et al. [59] used 3 IH neuron models (hypoxia duration: 2, 5, 10 min) to determine the appropriate hypoxia time to study autophagy, their results indicated that the hypoxia phase (1.5%O2) for 5 min and reoxygenation phase (21%O2) for 10 min led to significant difference on the markers of apoptosis and autophagy. When SD rats were treated with IH for 2, 4, and 6 weeks, the level of autophagy was increased in the hippocampus as the treatment time increased, but there were no significant differences between rats treated for 4 and 6 weeks [60].
Table 2.
Autophagy in the cognitive impairment of OSA model
| Year | PMID | IH model | Brain region | Autophagy markers (methods) | Regulation mechanism | Behavior test |
|---|---|---|---|---|---|---|
| 2019 | 31678553 |
C57BL/6 J mice, KKAy type 2 diabetes model mice: (O2 decreased from 21 to 5% over 30 s and then rebounded to 21% and was maintained 90 s) 5–21% O2, 30 cycles per hour, 8 h/d, 4 weeks HT22 cell: 1.5% O2 for 30 s and 21% O2 for 90 s, maintained 8 h |
Hippocampus/neuron |
LC3II/LC3I↓, P62↑, Beclin1↓ (WB and IF) |
HMGB1/TLR4 signaling pathway↑ | – |
| 2020 | 33176803 |
C57BL/6 J mice: O2 fluctuated from 21 to 5% with a period of 60 s, 8 h/d, 3 weeks BV2 cell: O2 fluctuated from 21 to 5%, maintained 24 h |
Hippocampus/microglia | BNIP3/NIX, BNIP3 ATG-7 and LC3II↑; P62, TOM20 ↓, MMP↓, mitochondrial fragmentation and swelling, and loss of cristae (WB, IF, flow cytometry analysis, TEM) | JNK-ERK signaling pathway↑ (p-JNK/JNK↑, p-ERK/ERK↑) | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
| 2020 | 31549780 | C57BL/6 J mice: an orbital rotor with a speed of 55 Hz and a repeated cycle of 10 s on, 110 s off, during the light‐on phase, continuously for 2 months | Cortex | LC3B↑, Beclin1↑ and UVRAG↑ (WB and IF) | – |
Morris water maze: escape latency↑, crossing number↓, dwell time↓; Novel object recognition test: discrimination index↓; Open field test: time in the central zone↓, total distance↑ |
| 2021 | 34469698 | SD rats: The compressed air and nitrogen were filled into the chamber alternately at 30 s intervals. O2 fluctuated from 21% to 7.5%, 8 h/d, 4 weeks | Hippocampus (CA1)/neuron | Autophagic vacuole↑, LC3I↓, LC3II↑, P62↓ (TEM, WB and IF) | p38MAPK signaling pathway↑(p-p38MAPK/MAPK↑) | Morris water maze: escape latency↑, crossing number↓, dwell time↓ |
| 2021 | 33717152 |
C57BL/6 mice: O2 fluctuated from 24 to 7% with a period of 60 s, 10 h/d, 5 weeks BV2 cell: O2 fluctuated from 21 to 5%, maintained 24 h |
Hippocampus/microglia | ATG-5, ATG-7, PINK1, Parkin, Beclin1 and LC3 II↑; P62, TOM20↓, MMP↓, mitochondrial swelling and loss of mitochondrial cristae (WB, IF, flow cytometry analysis, TEM) | NLRP3 | Contextual fear conditioning test: freezing times in the contextual and tone conditional↓ |
| 2021 | 34685469 | C57BL/6 mice: sleep was interrupted at 2 min intervals during the 12 h light period, maintained 5d | Striatum and hippocampus |
striatum: LC3II↑, Beclin1↑, P62↑ (WB); hippocampus: LC3II↑, Beclin1↓, P62↓ (WB) |
– | – |
| 2022 | 35401114 | C57BL/6 mice: O2 fluctuated from 21.0 ± 0.5% to 9 ± 1.5%, 90 s/cycle, 7 h/d, 4 weeks | Hippocampus (CA1)/neuron | Autophagosomes↑, LC3II/LC3I↑, P62↑, Beclin1↑, ATG5↑ (TEM, WB and IF) | – | Eight-arm maze: working memory errors↑, reference memory errors↑, total errors↑ |
| 2022 | 36611953 | C57BL/6 mice: sleep was interrupted at 2 min intervals during the 12 h light period, maintained for 3 weeks | Hippocampus | LC3II↑ (WB) | – | – |
| 2023 | 37268259 | HT22 cell: 0.1% O2 3 min and 21% O2 7 min for 6 cycles/h, 48 h | – |
Fluorescence intensity of autophagy↓, autophagosomes↓, LC3II/LC3I↓, P62↑, Beclin1↓ (autophagy staining test kit, TEM, WB) |
AMPK-mTOR signaling pathway (P-AMPK/AMPK↓, P-mTOR/mTOR↑) | – |
TEM transmission electron microscopy, WB western blot, IF immunofluorescence, MMP mitochondrial membrane potential
To date, the effects of IH on autophagy levels remain inconclusive, with inconsistent findings reported across different studies. IH could reduce the level of autophagy by altering the expression of adenosine 5′-monophosphate-activated protein kinase (AMPK)-mammalian target of rapamycin (mTOR) signaling pathway [42]. A study found autophagy was reduced both in normal mice and diabetes mice after IH treatment, the autophagy level was lower and could be regulated by HMGB1/TLR4 signaling in the diabetes model [53]. Moreover, Li et al. [61] detected that the expressions of LC3II/LC3I, P62, Beclin1, and autophagy-related genes (ATG) 5 were increased, and autophagosomes increased, which indicated autophagy flux inhibition in the hippocampus of IH mice. However, other studies show that IH could promote autophagy. In IH-treated SD rats, the level of autophagy activated, inhibiting the p38 mitogen-activated protein kinase (MAPK) signaling pathway could further activate autophagy in hippocampal nerve cells, thus reducing nerve cell injury [60]. In addition, IH was shown to aggravate CI in brain ischemia/reperfusion rat model, the underlying mechanism was associated with phosphatidylinositol 3‑kinase (PI3K)‑mTOR‑autophagy pathway activation and nerve cell damage [62]. The reason for the inconsistent results may be the different disease courses caused by the differences in IH conditions.
IH initiated time-dependent mitophagy to remove damaged mitochondria in microglia, which changed levels of inflammation and oxidative stress [63, 64]. Activated BCL2-interacting protein 3 (BNIP3)-dependent and PTEN-induced putative kinase1 (PINK1)-Parkin pathway-mediated mitophagy were detected in microglia of IH model, the former was activated by c-Jun N-terminal kinase (JNK)-extracellular regulated kinase (ERK) signaling pathway and the latter was regulated by nucleotide‐binding domain like receptor protein 3 (NLRP3) expression [63, 64].
SF is also involved in the CI of OSA through autophagy dysregulation. The unique autophagy rhythm of the hippocampus is altered by SF [65]. Short-term SF leads to dysregulated autophagy in the striatum and hippocampus but not in the frontal cortex, indicating that SF-related autophagy may tend to occur in specific brain regions [66]. Endosome-autophagosome-lysosome pathway dysfunction and microglia-mediated neuroinflammation were considered to be similar mechanisms for chronic SF and neurodegenerative diseases [67]. Short-term SF-activated microglia are in the striatum, while long-term SF-activated microglia are in the hippocampus [66, 68]. SF-induced microglial activation in the hippocampus was related to dysregulation of autophagy [68].
Ferroptosis
Ferroptosis is defined as an iron-dependent regulated necrosis that is caused by massive lipid peroxidation-mediated membrane damage, and mainly occurs through the extrinsic or transporter-dependent pathway and the intrinsic or enzyme-regulated pathway [69, 70]. It is implicated in the pathological cell death associated with many diseases including neurodegenerative diseases and ischemia–reperfusion injury [69]. However, the role of ferroptosis in OSA-CI is still in the preliminary stage of exploration.
The expression of ferroptosis-related genes and iron metabolism are altered in OSA patients. Serum iron levels in obese patients with OSA were significantly higher than those without OSA, and transferrin saturation correlated with OSA severity and duration of hypoxia, suggesting an association between OSA-induced hypoxia and iron metabolism in obese patients [71]. Huang et.al identified 13 ferroptosis-related differentially expressed genes in OSA patients with CPAP treatment as potential targets [72]. Recently, a large cohort study demonstrated that iron homeostasis was altered in the brain of OSA patients, increased iron accumulation was observed in specific cortical regions by quantitative MRI analysis, and nocturnal hypoxemia was closely related to higher iron levels [73]. On the other hand, malondialdehyde (MDA), 8-iso-prostaglandin F2α (8-iso-PGF2α), oxidized low-density lipoprotein (oxLDL), and thiobarbituric acid-reactive substances (TBARS) are common markers of lipid peroxidation in OSA [24, 74]. The central nervous system is rich in lipids, ROS can oxidize polyunsaturated fatty acids easily, and IH induces the increase of ROS and the imbalance of the oxidation-antioxidant system [24, 35, 75]. Therefore, lipid peroxidation may affect the cognition of OSA.
Similar to the MRI data of OSA patients, iron overload was found in the hippocampus of IH murine models, and neurological damage was attenuated when IH-induced ferritin and transferrin receptor 1 were inhibited [52]. Neuronal ferroptosis was also found in the prefrontal cortex of IH mice, which led to neuronal loss and cognitive decline [76]. One study examined ferroptosis-related markers in hippocampus of IH rat model and found mitochondrial damage, increased Fe2+, Malondialdehyde (MDA) levels, and Acyl-CoA synthetase long-chain family member 4 (ACSL4) protein expression were observed in the IH group, while superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase 4 (GPX4) protein expression were decreased [12].
Pyroptosis
Pyroptosis is a PCD mediated by gasdermin protein, which is triggered by caspases activated by some inflammasomes. It is manifested as cell swelling, plasma membrane cleavage, chromatin fragmentation, and the release of proinflammatory substances in cells [77, 78]. Cytokine release in pyroptosis induces cell injury, leads to cell damage and dysfunction of neurons and blood vessel cells, and results in loss of memory and executive function [79]. Ischemia/hypoxia can lead to pyroptosis of brain neurons, microglia, and microvascular endothelial cells [80–83]. However, the relationship between pyroptosis and IH in CI of OSA remains unclear.
A previous study showed that IH aggravated neuroinflammation and pyroptosis in early brain injury after subarachnoid hemorrhage through the Apoptosis-associated speck-like protein containing a CARD (ASC)/hypoxia-inducible factor-1α (HIF-1α) pathway [84]. Chen et al. [61] isolated exosomes from the plasma of severe OSA patients, coincubated mouse hippocampal neurons with exosomes, or injected exosomes into mice via caudal veins, their results suggested that OSA plasma-derived exosomes promoted neuronal pyroptosis and increased expression of inflammatory factors and led to cognitive dysfunction in mice [85]. The inflammasome, also known as the pyroptosome, is a supramolecular entity that initiates the pyroptotic cell death process [79]. NLRP3 is a member of the inflammasome family, and NLRP3 signaling is increased in a HIF-1α-dependent manner in severe OSA patients and IH monocytes, with upregulated inflammatory cytokines (interleukin-1β and interleukin-18) and gasdermin D [86]. Expression levels of pyroptosis protein including NLRP3, cleaved caspase-1, and ASC are also increased in the hippocampus of IH mice [64]. These studies indicate that pyroptosis may be involved in the progression of CI in OSA.
Interaction between PCDs
Different types of PCD can be considered as a unified cell death program, in which the individual pathways are highly linked and can be flexibly coordinated and compensated for each other [87]. Ferroptosis has been found to interact with apoptosis and autophagy in neurodegenerative diseases, the balance of PCDs can be controlled by regulating their same protein target [88]. In OSA, oxidative stress, ERS, and mitochondrial damage may be the common pathways regulating PCDs. Under the condition of oxidative stress, intricate interactions occur among apoptosis, autophagy, and ferroptosis. Cytoprotective autophagy contributes to cellular homeostasis by eliminating damaged organelles and misfolded protein aggregates, thereby reducing reactive oxygen species and mitigating apoptosis. Nevertheless, under specific pathological conditions, autophagy may transition into an apoptotic response. Ferroptosis inducers have been shown to activate apoptotic pathways via endoplasmic reticulum and mitochondrial stress and can also initiate non-selective autophagy. Moreover, excessive autophagy, in the presence of oxidative stress and lipid peroxidation, may facilitate ferroptosis. Selective and chaperone-mediated autophagy further potentiates ferroptosis by degrading key inhibitory proteins, leading to increased accumulation of intracellular free iron and lipid peroxides [89]. A recent study suggested that IH-induced neuronal ferroptosis mediates the occurrence of ERS [76]. Meanwhile, ERS can promote neuronal apoptosis under IH treatment [47, 48]. Furthermore, mitochondrial dysfunction or damage triggered by IH has been identified as an upstream pathway of apoptosis and ferroptosis [90, 91]. Few studies have investigated the interaction between PCDs under IH conditions. Activating the Nrf2 pathway and autophagy can protect the cardiac function of IH mice and reduce cardiomyocyte apoptosis [92, 93]. While inhibition of autophagy in liver kupffer cells could aggravate IH-induced apoptosis [94]. Ferrostatin-1, one of the ferroptosis inhibitors, was reported to be able to ameliorate apoptosis and injury in aortic endothelial cells [90] and lung cells [95] under IH condition. However, crosstalk between PCDs has not been fully discussed yet in OSA-CI. In IH treated neuron model, Tanshinone IIA could inhibit oxidative stress and neuronal apoptosis by activating autophagy [42]. In IH-treated mice, Sulforaphane alleviates hippocampal neuronal apoptosis by enhancing Nrf2 nuclear translocation and autophagy [61]. Notably, simultaneous inhibition of multiple PCDs is considered to have more potential to improve ischemia–reperfusion injury than a single type of PCD [96]. Therefore, it is worth exploring the links between different PCDs, which may have potential clinical value.
PCD induced by intercellular interactions
IH can directly activate microglia and promote the release of inflammatory cytokines in the central nervous system. Excessive nerve inflammation further promotes the activation of glial cells, causes synaptic loss, and neuron damage, and eventually leads to neurocognitive defects [11]. The majority of studies on PCD induced by intercellular interactions in OSA focused on apoptosis, neuronal apoptosis can be indirectly mediated by IH-induced microglial activation. Wang et al. found SUMO-specific proteases 1 (SENP1) expression was decreased in IH-treated microglia, which reduced the level of Target of Myb 1 (TOM1) and peroxisome proliferator-activated receptor γ (PPARγ) by promoting their SUMOylation, and aggravated neuroinflammation and neuronal apoptosis [97, 98]. Another study also focused on the effects of microglia on neurons and found that IH induced microglial activation and release of pro-inflammatory cytokines through the HMGB1/TLR4/NF-κB signaling pathway, leading to neuronal apoptosis [99]. Besides, the combined intervention of high glucose and IH can activate microglia, lead to the release of neuroinflammatory factors (ROS, TNF-α, IL-1β), and mediate the apoptosis of HT22 cells in co-culture through the PI3K/Akt/GSK-3β signaling pathway [100].
Possible treatment for OSA-CI
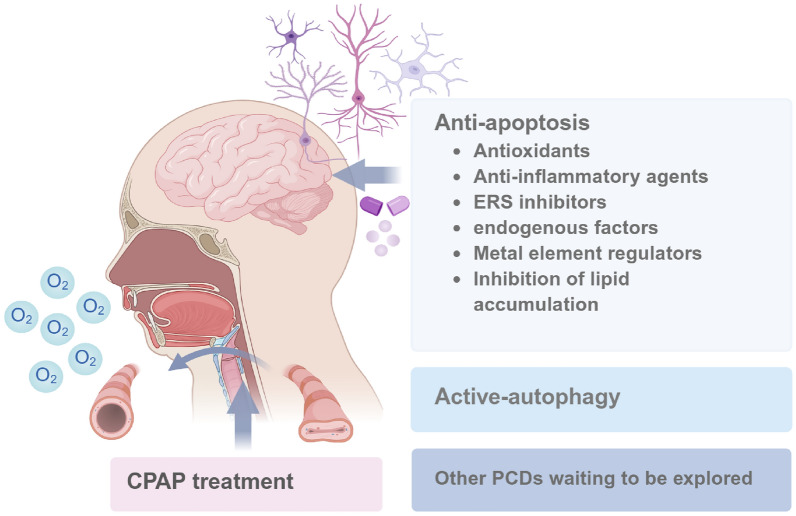
Most studies have been performed in animal or cellular models of IH because of limited access to brain specimens from OSA patients. In addition to traditional CPAP treatment, previous studies have figured out various drugs that protect brain tissues from IH-induced CI by targeting apoptosis and autophagy (Fig. 2).
Fig. 2.
Possible treatments for OSA-CI
CPAP treatment
The first-line treatment for OSA is continuous positive airway pressure (CPAP) [101]. Most current research on the treatment of OSA-CI has focused on CPAP, and the data for other treatments is pretty limited. Adherence to CPAP therapy seems to be able to slow and reverse the cognitive decline in OSA patients and reduce the risk of progression to dementia [102]. The duration of CPAP therapy is critical to the effect of cognitive improvement in OSA patients. Previous studies showed that one month of CPAP treatment could reduce the sleepiness of OSA patients and improve verbal episodic memory [103], 12 weeks of treatment also showed a positive effect on verbal memory [104], and three months of treatment led to significant improvements in episodic memory, short-term memory, and executive function [105]. A systematic review suggested that CPAP therapy should be persisted for at least 4 weeks to improve OSA-CI [106]. Scholars have reported the time trajectory of NOR test performance in IH mouse models. Switching to normoxia in the early stage of intermittent hypoxia intervention and over a specific period, the CI of mice is partially reversed instead of full recovery [40]. However, there is little evidence of the pathological improvement of CI in OSA by CPAP or normoxia restoration. Therefore, we cannot summarize the effect of similar treatments on the different PCDs of hippocampal nerve cells.
Anti-apoptosis treatments to manage OSA-CI
Anti-apoptosis is considered to be an effective way to improve CI in neurodegenerative diseases and brain injury [107–109]. At present, most of the neuroprotective substances found by the existing evidence act on hippocampal neuron apoptosis in IH models.
Antioxidants
IH induces an oxidation-antioxidant imbalance in OSA [24]. OSA-CI is strongly associated with neuronal damage in brain regions most sensitive to hypoxia and oxidative stress, such as the hippocampus and cerebral cortex regions [2, 42, 110]. Imaging data indicating changes in brain function were correlated with the apnea–hypopnea index and oxygen desaturation index in OSA patients [111]. The level of oxidative stress in the peripheral blood of OSA patients is closely related to hypoxic sleep parameters and cognitive scores [112, 113]. Increased oxidative stress in the brain has also been demonstrated in rodent models with IH treatment [52].
Improving oxidative stress can alleviate CI by reducing neuron loss [114, 115]. Many plant extracts and active ingredients of herbal medicines and novel compounds play an important role in anti-apoptosis and anti-oxidative stress, thereby reducing the nerve damage of IH. A plant-derived drug named apocynin was reported to inhibit NADPH oxidase activity and attenuate neuronal apoptosis in the hippocampus of IH rats [116]. Protocatechuic acid is a simple phenolic compound with antioxidant and anti-inflammatory effects. It was found that protocatechuic acid can lessen oxidative stress and apoptosis in the hippocampus of IH rats [117]. Astragalus was reported to protect against IH-induced hippocampal neuron apoptosis in rats with decreased HIF-1α expression [118]. Sulforaphane (Nrf2 agonist) was found to have a neuroprotective effect in the IH and SF mice model, which could reduce the apoptosis of hippocampal neurons by increasing the level of Nrf2 [35]. Sulforaphane could also alleviate neuronal apoptosis by activating autophagy [61]. Moreover, N-acetylcysteine can increase the level of thioredoxin, reduce neuronal apoptosis, and improve cognitive function in the hippocampus of IH rats [43]. Thus, Antioxidants have been shown to have neuroprotective effects in IH models.
Anti-inflammatory agents
The systemic inflammation of OSA is mediated by IH [119]. Peripheral inflammatory signals pass through the damaged blood–brain barrier and vagus nerve into the central nervous system, causing neuroinflammation [120]. Many studies have found that peripheral inflammation in OSA patients is associated with cognitive decline [112, 121, 122]. On the other hand, IH can directly activate microglia and astrocytes and stimulate excessive neuroinflammation, leading to synaptic damage and loss, and neuronal apoptosis [11].
Neuroprotective therapy based on anti-neuroinflammation is effective in many neurodegenerative diseases with cognitive decline [123–125]. It seems to be effective for OSA as well. Toll-like receptors (TLRs) family are the initiating molecules of inflammation, which are widely expressed in microglia, astrocytes, and neurons. The activation of glial cells in neuroinflammation is closely related to TLRs [126, 127]. Increased expression of TLR2 can stimulate glial cells to secrete inflammatory factors, ultimately leading to TLR2-induced neuronal apoptosis [128, 129]. TLR2 gene knockout could reduce neuron loss and abnormally activated glia in the hippocampus of IH mice [130]. Additionally, pseudo ginsenoside GQ was found to have anti-inflammatory effects, significantly ameliorated spatial learning deficits, and inhibited microglial activation, pro-inflammatory cytokine release, and neuronal apoptosis in the hippocampus of IH mice [99]. Recently, the application of Banxia-Houpu decoction showed an increase in the Bcl-2/Bax ratio, thereby mitigating neuronal damage in IH mice [131]. Moreover, SENP1 promotes microglia migration by alleviating SUMOylation of protein TOM1, reducing neuroinflammation, neuronal pathological deposition, and neuronal apoptosis subsequently [97].
ERS inhibitors
Misfolded/unfolded proteins within the endoplasmic reticulum are a common feature of nervous system diseases, inducting endoplasmic reticulum stress. It could initiate an unfolded protein response to maintain protein homeostasis, but cell death and inflammation will be activated if the damage is irreversible [132]. Previous studies have proposed that hypoxia elicits ERS [133]. ERS-mediated apoptosis is one of the important mechanisms of cognitive dysfunction in OSA [41].
Some drugs that can directly or indirectly inhibit ERS have attracted people’s attention. Tauroursodeoxycholate (TUDCA), which is a member of the ERS inhibitor, can reduce IH-induced neuronal apoptosis in the hippocampal CA1 region by upregulating the anti-apoptotic protein Bcl-2 in mice [46]. Treatment with CaSR inhibitors alleviates the apoptosis of hippocampal neurons in IH models by inhibiting the ERS pathway indirectly [48].
Endogenous factors
Some endogenous factors were proven to be involved in the apoptosis of hippocampal neurons under IH conditions. Orexin, a neuropeptide produced in the lateral hypothalamus, has attracted much attention in sleep disorders and the treatment of neurological diseases [134, 135]. Orexin A was reported to improve IH-induced hippocampal apoptosis and oxidative stress [136]. Hypoxia facilitates the accumulation of the presynaptic neuromodulator adenosine, which modulates synaptic plasticity through its interaction with the inhibitory adenosine 1 receptor (A1R) and facilitatory adenosine 2A receptor (A2AR). When mice were treated with IH, activation of adenosine A1R and blockade of adenosine A2AR reduced apoptosis of hippocampal neurons, attenuated long-term potentiation, and alleviated memory impairment [137, 138]. Melatonin was also found to have a protective effect against apoptosis of hippocampal neurons in IH rats [139].
Metal element regulators
Abnormal deposition/distribution of metal ions in different brain regions induces oxidative stress, endoplasmic reticulum stress, mitochondrial and autophagy dysfunction, and is involved in CI in a variety of neurodegenerative diseases [140, 141]. Emerging evidence also suggests that CI in OSA is related to the imbalance of metal homeostasis. Some scholars have detected abnormal iron accumulation in the brain of OSA patients through imaging [73]. Similarly, IH mice showed increased brain iron levels [131]. Iron may specifically contribute to nerve cell death when the level of glutathione is reduced [142]. In addition, treating mice with IH for a long time could increase brain cobalt, predominantly in the white matter [143].
Some chelating agents and metal-based drugs that regulate metal ion homeostasis have been proposed as one of the alternative treatment options for neurodegenerative diseases [144]. Several studies have set their sights on these unique therapeutic targets in OSA. Mood stabilizing agent LiCl decreased the activity of GSK-3β and increased the expression of β-catenin, and partially reversed neuronal apoptosis and spatial memory deficits in IH mice [51]. Huperzine A, which acts as an effective iron chelator, could attenuate apoptosis, oxidative stress, and synaptic plasticity mediated by IH in mice [52].
Inhibition of lipid accumulation
Lipid dysregulation can stimulate pathological protein deposition, and lead to mitochondrial and endoplasmic reticulum dysfunction and even nerve cell death. It can also increase the burden on the cerebrovascular system, induce insulin resistance, and thus affect the structure of neurons indirectly [145]. A recent study examined the changes in lipid metabolism in the cerebrospinal fluid and found that the primary mechanism for the association between OSA and Alzheimer’s disease may be an increase in lipid oxidation in the central nervous system.
It has been reported that cognition can be improved by regulating the lipids of nerve cells. Gedam et al. found that hypoxia in microglia can induce lipid droplet accumulation, while depletion of the C3a receptor complement saves the dysregulated lipid profile and improves the phagocytosis and aggregation ability of microglia [146]. An active component of Salvia miltiorrhiza, SMND-309, dramatically alleviated CI in mice by decreasing lipid droplet accumulation in the hippocampus, and this further reduced neuronal injury, neuroblast apoptosis, and glial activation [54].
Active-autophagy treatments to manage OSA-CI
Autophagy has been considered as a potential therapeutic target for neurodegenerative diseases according to available evidence [147, 148]. Autophagy and mitophagy are important potential targets for OSA neurocognitive impairment as well. Tanshinone IIA is extracted from salvia miltiorrhiza, which is a traditional Chinese medicine, it can inhibit oxidative stress and neuronal apoptosis by activating the AMPK/mTOR autophagy pathway under IH conditions [42]. Pinocembrin is a natural flavonoid drug, that has antimicrobial, anti-inflammatory, and antioxidant properties [149]. Pinocembrin can activate BNIP3-dependent mitophagy through the JNK-ERK pathway to exert neuroprotective function in IH models, inhibiting the formation of NLRP3 inflammasome [64], and NLRP3 deficiency was reported to protect against IH-mediated neuroinflammation and mitochondrial ROS [63].
Conclusion and prospects
CI is an important complication of OSA, which brings great inconvenience to the lives of patients [6]. CPAP can partially improve CI in OSA [106], but poor adherence drives us to explore new effective treatments. In this review, we briefly summarized the relevant studies that discussed the involvement of PCD in the CI of OSA. We found that IH-induced PCD had a critical effect on the mechanisms that produced the ultimate neurological deficit, and the PCD involved mainly included apoptosis, autophagy, ferroptosis, and pyroptosis. IH regulates PCD directly or through specific pathways, and drugs targeting related molecules have the potential to improve cognitive function.
In the future, the following contents are what we need to pay attention to in this field: (a) Current IH disease models (oxygen concentration, hypoxia duration, and hypoxia frequency and extent) need to be unified as much as possible, select the one that is more relevant to the clinical reality, and fully consider the role of SF, then describe PCD changes in different disease phases. (b) New and comprehensive PCDs deserve to be investigated to find out whether there is a dominant type of PCD in OSA-CI and to explore the deeper molecular mechanisms. (C) Reciprocal regulation between PCDs involved. (d) PCD-based therapy must be developed and evaluated in clinical trials.
Acknowledgements
All figures in this article were drawn using Biorender (https://www.biorender.com/).
Author contributions
D.Z., R.O., and Y.O. made the conceptualization. Y.O. conducted manuscript writing. X.W. helped visualization. D.Z. and R.O. took charge of funding acquisition and project administration. All authors reviewed and edited the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of Hunan Province (Grant No. 2024JJ5488), the Health Commission of Hunan Province (Grant No. W20243062), and the National Key Clinical Specialty Construction Projects of China.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dandan Zong, Email: zongdandan0402@csu.edu.cn.
Ruoyun Ouyang, Email: ouyangruoyun@csu.edu.cn.
References
- 1.Chang JL, et al. International consensus statement on obstructive sleep apnea. Int Forum Allergy Rhinol. 2023;13:1061–482. 10.1002/alr.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meliante PG, et al. Molecular pathology, oxidative stress, and biomarkers in obstructive sleep apnea. Int J Mol Sci. 2023;24:5478. 10.3390/ijms24065478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjafield AV, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–98. 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv R, et al. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct Target Ther. 2023;8:218. 10.1038/s41392-023-01496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pase MP, et al. Sleep architecture, obstructive sleep apnea, and cognitive function in adults. JAMA Netw Open. 2023;6:e2325152. 10.1001/jamanetworkopen.2023.25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanek J, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–8. 10.1016/j.sleep.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Park W, et al. Diversity and complexity of cell death: a historical review. Exp Mol Med. 2023;55:1573–94. 10.1038/s12276-023-01078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayagaki N, Webster JD, Newton K. Control of cell death in health and disease. Annu Rev Pathol. 2024;19:157–80. 10.1146/annurev-pathmechdis-051022-014433. [DOI] [PubMed] [Google Scholar]
- 9.Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28:2029–44. 10.1038/s41418-021-00814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dailah HG. Potential of therapeutic small molecules in apoptosis regulation in the treatment of neurodegenerative diseases: an updated review. Molecules. 2022;27:7207. 10.3390/molecules27217207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J Neuroinflammation. 2020;17:229. 10.1186/s12974-020-01905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z-L, et al. The role of ferroptosis in chronic intermittent hypoxia-induced cognitive impairment. Sleep Breath. 2023;27:1725–32. 10.1007/s11325-022-02760-6. [DOI] [PubMed] [Google Scholar]
- 13.Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev. 2018;38:39–49. 10.1016/j.smrv.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Davies CR, Harrington JJ. Impact of obstructive sleep apnea on neurocognitive function and impact of continuous positive air pressure. Sleep Med Clin. 2016;11:287–98. 10.1016/j.jsmc.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Tang S, Lyu X, Yang C, Chen X. Structural and functional brain alterations in obstructive sleep apnea: a multimodal meta-analysis. Sleep Med. 2019;54:195–204. 10.1016/j.sleep.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Paulekiene G, Pajarskiene M, Pajediene E, Radziunas A. Sleep dysfunction and grey matter volume. Curr Neurol Neurosci Rep. 2022;22:275–83. 10.1007/s11910-022-01190-x. [DOI] [PubMed] [Google Scholar]
- 17.Baril A-A, et al. Obstructive sleep apnea and the brain: a focus on gray and white matter structure. Curr Neurol Neurosci Rep. 2021;21:11. 10.1007/s11910-021-01094-2. [DOI] [PubMed] [Google Scholar]
- 18.Rostampour M, et al. White matter alterations in patients with obstructive sleep apnea: a systematic review of diffusion MRI studies. Sleep Med. 2020;75:236–45. 10.1016/j.sleep.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, et al. Selective microstructural integrity impairments of the anterior corpus callosum are associated with cognitive deficits in obstructive sleep apnea. Brain Behav. 2019;9:e01482. 10.1002/brb3.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M-H, et al. Altered structural brain network resulting from white matter injury in obstructive sleep apnea. Sleep. 2019;42:zsz120. 10.1093/sleep/zsz120. [DOI] [PubMed] [Google Scholar]
- 21.Tummala S, et al. Global and regional brain non-gaussian diffusion changes in newly diagnosed patients with obstructive sleep apnea. Sleep. 2016;39:51–7. 10.5665/sleep.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang N, et al. Microstructural brain abnormalities and associated neurocognitive dysfunction in obstructive sleep apnea: a pilot study with diffusion kurtosis imaging. J Clin Sleep Med. 2024;20:1571–8. 10.5664/jcsm.11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147:266–74. 10.1378/chest.14-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Zhou H, Liu H, Xu P. Role of oxidative stress in the occurrence and development of cognitive dysfunction in patients with obstructive sleep apnea syndrome. Mol Neurobiol. 2023. 10.1007/s12035-023-03899-3. [DOI] [PubMed] [Google Scholar]
- 25.Teleanu DM, et al. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. 2022;23:5938. 10.3390/ijms23115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takata F, Nakagawa S, Matsumoto J, Dohgu S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front Cell Neurosci. 2021;15:661838. 10.3389/fncel.2021.661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polsek D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev. 2018;86:142–9. 10.1016/j.neubiorev.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frisoni GB, et al. The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat Rev Neurosci. 2022;23:53–66. 10.1038/s41583-021-00533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93:1778–94. 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- 30.Shiota S, et al. Chronic intermittent hypoxia/reoxygenation facilitate amyloid-β generation in mice. J Alzheimers Dis. 2013;37:325–33. 10.3233/JAD-130419. [DOI] [PubMed] [Google Scholar]
- 31.Frost B. Alzheimer’s disease and related tauopathies: disorders of disrupted neuronal identity. Trends Neurosci. 2023;46:797–813. 10.1016/j.tins.2023.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alomri RM, Kennedy GA, Wali SO, Ahejaili F, Robinson SR. Differential associations of hypoxia, sleep fragmentation, and depressive symptoms with cognitive dysfunction in obstructive sleep apnea. Sleep. 2021;44:zsaa213. 10.1093/sleep/zsaa213. [DOI] [PubMed] [Google Scholar]
- 33.Yan L, et al. Altered regional cerebral blood flow in obstructive sleep apnea is associated with sleep fragmentation and oxygen desaturation. J Cereb Blood Flow Metab. 2021;41:2712–24. 10.1177/0271678X211012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, et al. Alzheimer’s disease biomarkers and complement proteins mediate the impact of sleep fragmentation on cognitive impairment in obstructive sleep apnea patients without dementia. J Alzheimers Dis. 2023;95:1685–96. 10.3233/JAD-221288. [DOI] [PubMed] [Google Scholar]
- 35.Qiu X, et al. The protective role of Nrf2 on cognitive impairment in chronic intermittent hypoxia and sleep fragmentation mice. Int Immunopharmacol. 2023;116:109813. 10.1016/j.intimp.2023.109813. [DOI] [PubMed] [Google Scholar]
- 36.Nair D, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184:1305–12. 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puech C, et al. Cognitive impairments, neuroinflammation and blood-brain barrier permeability in mice exposed to chronic sleep fragmentation during the daylight period. Int J Mol Sci. 2023;24:9880. 10.3390/ijms24129880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ketelut-Carneiro N, Fitzgerald KA. Apoptosis, pyroptosis, and necroptosis-oh my! The many ways a cell can die. J Mol Biol. 2022;434:167378. 10.1016/j.jmb.2021.167378. [DOI] [PubMed] [Google Scholar]
- 39.Shoshan-Barmatz V, Arif T, Shteinfer-Kuzmine A. Apoptotic proteins with non-apoptotic activity: expression and function in cancer. Apoptosis. 2023;28:730–53. 10.1007/s10495-023-01835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gozal D, Khalyfa A, Qiao Z, Almendros I, Farré R. Temporal trajectories of novel object recognition performance in mice exposed to intermittent hypoxia. Eur Respir J. 2017;50:1701456. 10.1183/13993003.01456-2017. [DOI] [PubMed] [Google Scholar]
- 41.Cai X-H, et al. Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp Neurol. 2014;257:148–56. 10.1016/j.expneurol.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Si J, et al. Tanshinone IIA inhibited intermittent hypoxia induced neuronal injury through promoting autophagy via AMPK-mTOR signaling pathway. J Ethnopharmacol. 2023;315:116677. 10.1016/j.jep.2023.116677. [DOI] [PubMed] [Google Scholar]
- 43.Yang X-H, Liu H-G, Liu X, Chen J-N. Thioredoxin and impaired spatial learning and memory in the rats exposed to intermittent hypoxia. Chin Med J. 2012;125:3074–80. [PubMed] [Google Scholar]
- 44.Huang Y, et al. TGF-β3 protects neurons against intermittent hypoxia-induced oxidative stress and apoptosis through activation of the Nrf-2/KEAP1/HO-1 pathway via binding to TGF-βRI. Neurochem Res. 2023;48:2808–25. 10.1007/s11064-023-03942-8. [DOI] [PubMed] [Google Scholar]
- 45.Merighi A, Lossi L. Endoplasmic reticulum stress signaling and neuronal cell death. Int J Mol Sci. 2022;23:15186. 10.3390/ijms232315186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L-H, et al. Critical role of endoplasmic reticulum stress in chronic intermittent hypoxia-induced deficits in synaptic plasticity and long-term memory. Antioxid Redox Signal. 2015;23:695–710. 10.1089/ars.2014.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L, et al. Suppression of CHOP reduces neuronal apoptosis and rescues cognitive impairment induced by intermittent hypoxia by inhibiting bax and bak activation. Neural Plast. 2021;2021:4090441. 10.1155/2021/4090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You C, et al. Blockage of calcium-sensing receptor improves chronic intermittent hypoxia-induced cognitive impairment by PERK-ATF4-CHOP pathway. Exp Neurol. 2023;368:114500. 10.1016/j.expneurol.2023.114500. [DOI] [PubMed] [Google Scholar]
- 49.Ying H, et al. Inhibition of calcium-sensing receptor alleviates chronic intermittent hypoxia-induced cognitive dysfunction via CaSR-PKC-ERK1/2 pathway. Mol Neurobiol. 2023;60:2099–115. 10.1007/s12035-022-03189-4. [DOI] [PubMed] [Google Scholar]
- 50.Zhu J, et al. Role of the Nrdp1 in brain injury induced by chronic intermittent hypoxia in rats via regulating the protein levels of ErbB3. Neurotox Res. 2020;38:124–32. 10.1007/s12640-020-00195-z. [DOI] [PubMed] [Google Scholar]
- 51.Pan Y-Y, et al. Altered Wnt signaling pathway in cognitive impairment caused by chronic intermittent hypoxia: focus on glycogen synthase kinase-3β and β-catenin. Chin Med J. 2016;129:838–45. 10.4103/0366-6999.178969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An J-R, et al. Huperzine A, reduces brain iron overload and alleviates cognitive deficit in mice exposed to chronic intermittent hypoxia. Life Sci. 2020;250:117573. 10.1016/j.lfs.2020.117573. [DOI] [PubMed] [Google Scholar]
- 53.Guo X, et al. HMGB1/TLR4 promotes apoptosis and reduces autophagy of hippocampal neurons in diabetes combined with OSA. Life Sci. 2019;239:117020. 10.1016/j.lfs.2019.117020. [DOI] [PubMed] [Google Scholar]
- 54.Li D, et al. Abnormal lipid droplets accumulation induced cognitive deficits in obstructive sleep apnea syndrome mice via JNK/SREBP/ACC pathway but not through PDP1/PDC pathway. Mol Med. 2022;28:3. 10.1186/s10020-021-00427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puech C, Badran M, Barrow MB, Runion AR, Gozal D. Solriamfetol improves chronic sleep fragmentation-induced increases in sleep propensity and ameliorates explicit memory in male mice. Sleep. 2023;46:zsad057. 10.1093/sleep/zsad057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleming A, et al. The different autophagy degradation pathways and neurodegeneration. Neuron. 2022;110:935–66. 10.1016/j.neuron.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlemmer F, et al. Beclin-1 increases with obstructive sleep apnea severity. Sleep Med. 2021;81:474–6. 10.1016/j.sleep.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y-C, et al. Autophagy impairment in patients with obstructive sleep apnea modulates intermittent hypoxia-induced oxidative stress and cell apoptosis via hypermethylation of the ATG5 gene promoter region. Eur J Med Res. 2023;28:82. 10.1186/s40001-023-01051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song S, Tan J, Miao Y, Zhang Q. Effect of different levels of intermittent hypoxia on autophagy of hippocampal neurons. Sleep Breath. 2017;21:791–8. 10.1007/s11325-017-1512-7. [DOI] [PubMed] [Google Scholar]
- 60.He Y, Liu Z, Huang Y, Li B. Role of the p38MAPK signaling pathway in hippocampal neuron autophagy in rats with chronic intermittent hypoxia. J Neurophysiol. 2021;126:1112–21. 10.1152/jn.00240.2021. [DOI] [PubMed] [Google Scholar]
- 61.Li X, et al. Sulforaphane attenuates chronic intermittent hypoxia-induced brain damage in mice via augmenting Nrf2 nuclear translocation and autophagy. Front Cell Neurosci. 2022;16:827527. 10.3389/fncel.2022.827527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo X, et al. Role of the PI3K-mTOR autophagy pathway in nerve damage in rats with intermittent hypoxia-aggravated whole brain ischemia. Mol Med Rep. 2019;20:1411–7. 10.3892/mmr.2019.10337. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, et al. NLRP3 deficiency protects against intermittent hypoxia-induced neuroinflammation and mitochondrial ROS by promoting the PINK1-parkin pathway of mitophagy in a murine model of sleep apnea. Front Immunol. 2021;12:628168. 10.3389/fimmu.2021.628168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gong L-J, Wang X-Y, Gu W-Y, Wu X. Pinocembrin ameliorates intermittent hypoxia-induced neuroinflammation through BNIP3-dependent mitophagy in a murine model of sleep apnea. J Neuroinflammation. 2020;17:337. 10.1186/s12974-020-02014-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He Y, et al. Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiol Int. 2016;33:553–60. 10.3109/07420528.2015.1137581. [DOI] [PubMed] [Google Scholar]
- 66.Cheng Y, Kim W-K, Wellman LL, Sanford LD, Guo M-L. Short-term sleep fragmentation dysregulates autophagy in a brain region-specific manner. Life. 2021;11:1098. 10.3390/life11101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie Y, et al. Chronic sleep fragmentation shares similar pathogenesis with neurodegenerative diseases: endosome-autophagosome-lysosome pathway dysfunction and microglia-mediated neuroinflammation. CNS Neurosci Ther. 2020;26:215–27. 10.1111/cns.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo L, et al. Sleep-disturbance-induced microglial activation involves CRH-mediated galectin 3 and autophagy dysregulation. Cells. 2022;12:160. 10.3390/cells12010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stockwell BR, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85. 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25. 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Tallec-Estève N, Rousseau C, Desrues B, Loréal O, Thibault R. Transferrin saturation is independently associated with the severity of obstructive sleep apnea syndrome and hypoxia among obese subjects. Clin Nutr. 2021;40:608–14. 10.1016/j.clnu.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Huang J, et al. Ferroptosis-related genes are considered as potential targets for CPAP treatment of obstructive sleep apnea. Front Neurol. 2023;14:1320954. 10.3389/fneur.2023.1320954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marchi NA, et al. Abnormal brain iron accumulation in obstructive sleep apnea: a quantitative MRI study in the HypnoLaus cohort. J Sleep Res. 2022;31:e13698. 10.1111/jsr.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pau MC, et al. Circulating malondialdehyde concentrations in obstructive sleep apnea (OSA): a systematic review and meta-analysis with meta-regression. Antioxidants. 2021;10:1053. 10.3390/antiox10071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prabhakar NR, Peng Y-J, Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J Clin Invest. 2020;130:5042–51. 10.1172/JCI137560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhong P, et al. Neuronal ferroptosis and ferroptosis-mediated endoplasmic reticulum stress: implications in cognitive dysfunction induced by chronic intermittent hypoxia in mice. Int Immunopharmacol. 2024;138:112579. 10.1016/j.intimp.2024.112579. [DOI] [PubMed] [Google Scholar]
- 77.Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–21. 10.1038/s41423-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu P, et al. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maiese K. The impact of aging and oxidative stress in metabolic and nervous system disorders: programmed cell death and molecular signal transduction crosstalk. Front Immunol. 2023;14:1273570. 10.3389/fimmu.2023.1273570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park CH, Park JY, Cho WG. Chemical hypoxia induces pyroptosis in neuronal cells by caspase-dependent gasdermin activation. Int J Mol Sci. 2024;25:2185. 10.3390/ijms25042185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, et al. ChemR23 activation attenuates cognitive impairment in chronic cerebral hypoperfusion by inhibiting NLRP3 inflammasome-induced neuronal pyroptosis. Cell Death Dis. 2023;14:721. 10.1038/s41419-023-06237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han C, Zhai L, Shen H, Wang J, Guan Q. Advanced glycation end-products (AGEs) promote endothelial cell pyroptosis under cerebral ischemia and hypoxia via HIF-1α-RAGE-NLRP3. Mol Neurobiol. 2023;60:2355–66. 10.1007/s12035-023-03228-8. [DOI] [PubMed] [Google Scholar]
- 83.Tan L-L, et al. TP53-induced glycolysis and apoptosis regulator alleviates hypoxia/ischemia-induced microglial pyroptosis and ischemic brain damage. Neural Regen Res. 2021;16:1037–43. 10.4103/1673-5374.300453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu J, et al. Obstructive sleep apnea aggravates neuroinflammation and pyroptosis in early brain injury following subarachnoid hemorrhage via ASC/HIF-1α pathway. Neural Regen Res. 2022;17:2537–43. 10.4103/1673-5374.339000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z, et al. Obstructive sleep apnea plasma-derived exosomes mediate cognitive impairment through hippocampal neuronal cell pyroptosis. Am J Geriatr Psychiatry. 2024. 10.1016/j.jagp.2024.01.017. [DOI] [PubMed] [Google Scholar]
- 86.Díaz-García E, et al. Inflammasome activation: a keystone of proinflammatory response in obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205:1337–48. 10.1164/rccm.202106-1445OC. [DOI] [PubMed] [Google Scholar]
- 87.Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21:678–95. 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 88.Dang X, et al. Correlation of ferroptosis and other types of cell death in neurodegenerative diseases. Neurosci Bull. 2022;38:938–52. 10.1007/s12264-022-00861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang B, et al. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023;97:1439–51. 10.1007/s00204-023-03476-6. [DOI] [PubMed] [Google Scholar]
- 90.Chen J, et al. Ferrostatin-1 reversed chronic intermittent hypoxia-induced ferroptosis in aortic endothelial cells via reprogramming mitochondrial function. Nat Sci Sleep. 2024;16:401–11. 10.2147/NSS.S442186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moulin S, et al. Intermittent hypoxia-induced cardiomyocyte death is mediated by HIF-1 dependent MAM disruption. Antioxidants. 2022;11:1462. 10.3390/antiox11081462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, et al. Alpha-lipoic acid alleviated intermittent hypoxia-induced myocardial injury in mice by promoting autophagy through Nrf2 signaling pathway. Eur J Pharmacol. 2025;994:177380. 10.1016/j.ejphar.2025.177380. [DOI] [PubMed] [Google Scholar]
- 93.Chang J-C, et al. Intermittent hypoxia induces autophagy to protect cardiomyocytes from endoplasmic reticulum stress and apoptosis. Front Physiol. 2019;10:995. 10.3389/fphys.2019.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, et al. CX3CL1 represses autophagy via CX3CR1/ CaMKIIδ/HDAC4/Rubicon axis and exacerbates chronic intermittent hypoxia induced Kupffer cell apoptosis. Cell Signal. 2023;111: 110873. 10.1016/j.cellsig.2023.110873. [DOI] [PubMed] [Google Scholar]
- 95.Chen J, et al. The role of ferroptosis in chronic intermittent hypoxia-induced lung injury. BMC Pulm Med. 2022;22:488. 10.1186/s12890-022-02262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian J, et al. Combination of emricasan with ponatinib synergistically reduces ischemia/reperfusion injury in rat brain through simultaneous prevention of apoptosis and necroptosis. Transl Stroke Res. 2018;9:382–92. 10.1007/s12975-017-0581-z. [DOI] [PubMed] [Google Scholar]
- 97.Wang H, et al. SENP1 modulates chronic intermittent hypoxia-induced inflammation of microglia and neuronal injury by inhibiting TOM1 pathway. Int Immunopharmacol. 2023;119:110230. 10.1016/j.intimp.2023.110230. [DOI] [PubMed] [Google Scholar]
- 98.Wang H, et al. Depletion of SENP1-mediated PPARγ SUMOylation exaggerates intermittent hypoxia-induced cognitive decline by aggravating microglia-mediated neuroinflammation. Aging. 2021;13:15240–54. 10.18632/aging.203084. [DOI] [PMC free article] [PubMed]
- 99.Tian Y, et al. Pseudoginsenoside GQ mitigates chronic intermittent hypoxia-induced cognitive damage by modulating microglia polarization. Int Immunopharmacol. 2024;126:111234. 10.1016/j.intimp.2023.111234. [DOI] [PubMed] [Google Scholar]
- 100.Shi Y, et al. DNA binding protein HMGB1 secreted by activated microglia promotes the apoptosis of hippocampal neurons in diabetes complicated with OSA. Brain Behav Immun. 2018;73:482–92. 10.1016/j.bbi.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 101.Donovan LM, et al. Strategies to assess the effect of continuous positive airway pressure on long-term clinically important outcomes among patients with symptomatic obstructive sleep apnea: an official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2023;20:931–43. 10.1513/AnnalsATS.202303-258ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seda G, Matwiyoff G, Parrish JS. Effects of obstructive sleep apnea and CPAP on cognitive function. Curr Neurol Neurosci Rep. 2021;21:32. 10.1007/s11910-021-01123-0. [DOI] [PubMed] [Google Scholar]
- 103.Rosenzweig I, et al. Changes in neurocognitive architecture in patients with obstructive sleep apnea treated with continuous positive airway pressure. EBioMedicine. 2016;7:221–9. 10.1016/j.ebiom.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoyos CM, et al. continuous positive airway pressure for cognition in sleep apnea and mild cognitive impairment: a pilot randomized crossover clinical trial. Am J Respir Crit Care Med. 2022;205:1479–82. 10.1164/rccm.202111-2646LE. [DOI] [PubMed] [Google Scholar]
- 105.Dalmases M, et al. Effect of CPAP on Cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148:1214–23. 10.1378/chest.15-0171. [DOI] [PubMed] [Google Scholar]
- 106.Wang G, et al. Therapeutic effects of CPAP on cognitive impairments associated with OSA. J Neurol. 2020;267:2823–8. 10.1007/s00415-019-09381-2. [DOI] [PubMed] [Google Scholar]
- 107.Tsai Y-T, Kao S-T, Cheng C-Y. Medicinal herbs and their derived ingredients protect against cognitive decline in in vivo models of Alzheimer’s disease. Int J Mol Sci. 2022;23:11311. 10.3390/ijms231911311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guan P-P, Wang P. Integrated communications between cyclooxygenase-2 and Alzheimer’s disease. FASEB J. 2019;33:13–33. 10.1096/fj.201800355RRRR. [DOI] [PubMed] [Google Scholar]
- 109.Akamatsu Y, Hanafy KA. Cell death and recovery in traumatic brain injury. Neurotherapeutics. 2020;17:446–56. 10.1007/s13311-020-00840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang C, Zhou Y, Liu H, Xu P. The role of inflammation in cognitive impairment of obstructive sleep apnea syndrome. Brain Sci. 2022;12:1303. 10.3390/brainsci12101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou L, et al. Reduced regional homogeneity and neurocognitive impairment in patients with moderate-to-severe obstructive sleep apnea. Sleep Med. 2020;75:418–27. 10.1016/j.sleep.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 112.Zong D, Liu X, Shen C, Liu T, Ouyang R. Involvement of Galectin-3 in neurocognitive impairment in obstructive sleep apnea via regulating inflammation and oxidative stress through NLRP3. Sleep Med. 2023;101:1–10. 10.1016/j.sleep.2022.09.018. [DOI] [PubMed] [Google Scholar]
- 113.Zhou L, et al. Dysfunction of Nrf2-ARE signaling pathway: potential pathogenesis in the development of neurocognitive impairment in patients with moderate to severe obstructive sleep apnea-hypopnea syndrome. Oxid Med Cell Longev. 2018;2018:3529709. 10.1155/2018/3529709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X-S, et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br J Pharmacol. 2021;178:1114–32. 10.1111/bph.15346. [DOI] [PubMed] [Google Scholar]
- 115.Zhang R, et al. NOX2-derived hydrogen peroxide impedes the AMPK/Akt-mTOR signaling pathway contributing to cell death in neuronal cells. Cell Signal. 2022;94:110330. 10.1016/j.cellsig.2022.110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hui-guo L, Kui L, Yan-ning Z, Yong-jian X. Apocynin attenuate spatial learning deficits and oxidative responses to intermittent hypoxia. Sleep Med. 2010;11:205–12. 10.1016/j.sleep.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 117.Yin X, et al. Protocatechuic acid ameliorates neurocognitive functions impairment induced by chronic intermittent hypoxia. Sci Rep. 2015;5:14507. 10.1038/srep14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Q, et al. Protective effects of astragalus extract against intermittent hypoxia-induced hippocampal neurons impairment in rats. Chin Med J. 2013;126:1551–4. [PubMed] [Google Scholar]
- 119.Alterki A, Abu-Farha M, Al Shawaf E, Al-Mulla F, Abubaker J. Investigating the relationship between obstructive sleep apnoea, inflammation and cardio-metabolic diseases. Int J Mol Sci. 2023;24:6807. 10.3390/ijms24076807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tan S, Chen W, Kong G, Wei L, Xie Y. Peripheral inflammation and neurocognitive impairment: correlations, underlying mechanisms, and therapeutic implications. Front Aging Neurosci. 2023;15:1305790. 10.3389/fnagi.2023.1305790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dandan Z, et al. IL-33/ST2 mediating systemic inflammation and neuroinflammation through NF-kB participated in the neurocognitive impairment in obstructive sleep apnea. Int Immunopharmacol. 2023;115:109604. 10.1016/j.intimp.2022.109604. [DOI] [PubMed] [Google Scholar]
- 122.Xue S, et al. Complement activation mainly mediates the association of heart rate variability and cognitive impairment in adults with obstructive sleep apnea without dementia. Sleep. 2023;46:zsac146. 10.1093/sleep/zsac146. [DOI] [PubMed] [Google Scholar]
- 123.Zhang X-W, et al. Neuroinflammation inhibition by small-molecule targeting USP7 noncatalytic domain for neurodegenerative disease therapy. Sci Adv. 2022;8:eabo0789. 10.1126/sciadv.abo0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He X, et al. Intelligent lesion blood-brain barrier targeting nano-missiles for Alzheimer’s disease treatment by anti-neuroinflammation and neuroprotection. Acta Pharm Sin B. 2022;12:1987–99. 10.1016/j.apsb.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dong Y, Tang L. Microglial calcium homeostasis modulator 2: novel anti-neuroinflammation target for the treatment of neurodegenerative diseases. Neurosci Bull. 2023. 10.1007/s12264-023-01153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoffmann O, et al. TLR2 mediates neuroinflammation and neuronal damage. J Immunol. 2007;178:6476–81. [DOI] [PubMed] [Google Scholar]
- 127.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–63. 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 128.Ziegler G, et al. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–9. [DOI] [PubMed] [Google Scholar]
- 129.Ziegler G, et al. Blocking TLR2 in vivo protects against accumulation of inflammatory cells and neuronal injury in experimental stroke. J Cereb Blood Flow Metab. 2011;31:757–66. 10.1038/jcbfm.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li W, et al. TLR2 deficiency attenuated chronic intermittent hypoxia-induced neurocognitive deficits. Int Immunopharmacol. 2020;81:106284. 10.1016/j.intimp.2020.106284. [DOI] [PubMed] [Google Scholar]
- 131.Yang X-Y, et al. Banxia-Houpu decoction inhibits iron overload and chronic intermittent hypoxia-induced neuroinflammation in mice. J Ethnopharmacol. 2024;318:117078. 10.1016/j.jep.2023.117078. [DOI] [PubMed] [Google Scholar]
- 132.Shi M, Chai Y, Zhang J, Chen X. Endoplasmic reticulum stress-associated neuronal death and innate immune response in neurological diseases. Front Immunol. 2021;12:794580. 10.3389/fimmu.2021.794580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Akman M, et al. Hypoxia, endoplasmic reticulum stress and chemoresistance: dangerous liaisons. J Exp Clin Cancer Res. 2021;40:28. 10.1186/s13046-020-01824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dong P, et al. The potential role of the orexin system in premenstrual syndrome. Front Endocrinol. 2023;14:1266806. 10.3389/fendo.2023.1266806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shen Y-C, et al. Roles of neuropeptides in sleep-wake regulation. Int J Mol Sci. 2022;23:4599. 10.3390/ijms23094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu J, et al. Orexin A improves the cognitive impairment induced by chronic intermittent hypoxia in mice. Brain Res Bull. 2021;173:203–10. 10.1016/j.brainresbull.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y, et al. Neuroprotective effects of adenosine A1 receptor signaling on cognitive impairment induced by chronic intermittent hypoxia in mice. Front Cell Neurosci. 2020;14:202. 10.3389/fncel.2020.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X-C, et al. Blockade of adenosine A2A receptor alleviates cognitive dysfunction after chronic exposure to intermittent hypoxia in mice. Exp Neurol. 2022;350:113929. 10.1016/j.expneurol.2021.113929. [DOI] [PubMed] [Google Scholar]
- 139.Hung M-W, Tipoe GL, Poon AM-S, Reiter RJ, Fung M-L. Protective effect of melatonin against hippocampal injury of rats with intermittent hypoxia. J Pineal Res. 2008;44:214–21. 10.1111/j.1600-079X.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- 140.Mateo D, Marquès M, Torrente M. Metals linked with the most prevalent primary neurodegenerative dementias in the elderly: a narrative review. Environ Res. 2023;236:116722. 10.1016/j.envres.2023.116722. [DOI] [PubMed] [Google Scholar]
- 141.Wang L, et al. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl Neurodegener. 2020;9:10. 10.1186/s40035-020-00189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maher P. Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: implications for age-related neurodegenerative diseases. Free Radic Biol Med. 2018;115:92–104. 10.1016/j.freeradbiomed.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 143.Veasey SC, et al. Long-term intermittent hypoxia elevates cobalt levels in the brain and injures white matter in adult mice. Sleep. 2013;36:1471–81. 10.5665/sleep.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sales TA, et al. Recent developments in metal-based drugs and chelating agents for neurodegenerative diseases treatments. Int J Mol Sci. 2019;20:1829. 10.3390/ijms20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tong B, et al. Targeting dysregulated lipid metabolism for the treatment of Alzheimer’s disease and Parkinson’s disease: current advancements and future prospects. Neurobiol Dis. 2024;196:106505. 10.1016/j.nbd.2024.106505. [DOI] [PubMed] [Google Scholar]
- 146.Gedam M, et al. Complement C3aR depletion reverses HIF-1α-induced metabolic impairment and enhances microglial response to Aβ pathology. J Clin Invest. 2023;133:e167501. 10.1172/JCI167501. [DOI] [PMC free article] [PubMed]
- 147.Kim A, et al. New avenues for the treatment of Huntington’s disease. Int J Mol Sci. 2021;22:8363. 10.3390/ijms22168363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang Z, Yang X, Song Y-Q, Tu J. Autophagy in Alzheimer’s disease pathogenesis: therapeutic potential and future perspectives. Ageing Res Rev. 2021;72:101464. 10.1016/j.arr.2021.101464. [DOI] [PubMed] [Google Scholar]
- 149.Borriello M, Iannuzzi C, Sirangelo I. Pinocembrin protects from AGE-induced cytotoxicity and inhibits non-enzymatic glycation in human insulin. Cells. 2019;8:385. 10.3390/cells8050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.