Summary
Inadequate sleep in older adults is linked to health issues such as frailty, cognitive impairment and cardiovascular disorders. Maintaining regular sleep patterns is important for healthy aging, making effective sleep monitoring essential. While polysomnography is the gold‐standard for diagnosing sleep disorders, its regular use in home settings is limited. Alternative objective monitoring methods in the home can offer insights into natural sleep patterns and factors affecting them without the limitations of polysomnography. This scoping review aims to examine current technologies, sensors and sleep parameters used for home‐based sleep monitoring in older adults. It also aims to explore various predictors and outcomes associated with sleep to understand the factors of sleep monitoring at home. We identified 54 relevant articles using PubMed, Scopus, Web of Science and an AI tool (Research Rabbit), with 48 studies using wearable technologies and eight studies using non‐wearable technologies. Further, six types of sensors were utilized. The most common technology employed was actigraphy wearables, while ballistocardiography and electroencephalography were less common. The most frequent objective parameters of sleep measured were total sleep time, wakeup after sleep onset and sleep efficiency, with only six studies evaluating sleep architecture in terms of sleep stages. Additionally, six categories of predictors and outcomes associated with sleep were analysed, including Health‐related, Environmental, Interventional, Behavioural, Time and Place, and Social associations. These associations correlate with total sleep time, wakeup after sleep onset and sleep efficiency, and include in‐bed behaviours, exterior housing conditions, aerobic exercise, living place, relationship status, and seasonal thermal environments.
Keywords: actigraphy, healthy aging, objective sleep monitoring, sensors, sleep, technology
1. INTRODUCTION
Sleep is a critical and modifiable factor that has a profound impact on health and wellbeing in older adults (Ravyts & Dzierzewski, 2022). As individuals age, they may experience a decline in both the quality and quantity of sleep, irrespective of the presence of sleep disorders (Ancoli‐Israel, 2009). With aging, older adults might encounter increased sleep‐onset latency (SOL) and wake after sleep onset (WASO), and a decrease in total sleep time (TST), sleep efficiency (SE), slow‐wave sleep and rapid eye movement (REM) sleep (Espiritu, 2008). Several studies have demonstrated the negative association between sleep and various health outcomes in this population, including frailty (Sun et al., 2020), cognitive function (Behrens et al., 2023; Miyata et al., 2013), cardiovascular disorders (von Ruesten et al., 2012) and other age‐related conditions (Teramoto et al., 2005). These outcomes can directly or indirectly affect the wellbeing of older adults. The World Health Organization (WHO) referred to wellbeing in older age as healthy aging. The WHO claims it to be “a process of developing and maintaining the functional ability that enables wellbeing in older age” (Fallon & Karlawish, 2019). Given these facts, effective sleep monitoring becomes important to maintain functional ability, thus promoting healthy aging (Ravyts & Dzierzewski, 2022) and human longevity (Mazzotti et al., 2014) in older adults.
Sleep monitoring can be performed using either subjective measures or objectively. Subjective monitoring is the self‐perception where the individuals provide information about their sleep based on their own experience. Objective sleep evaluation involves the use of specialized sleep‐monitoring devices to monitor and analyse one or more biological signals of an individual, which helps to evaluate the structure and status of their sleep (Chen et al., 2023).
Evidence suggests that objective and subjective assessments of sleep quality may differ, especially in older adults and/or in people with cognitive impairment (Hita‐Yañez et al., 2013; O'Donnell et al., 2009). Subjective assessments rely mostly on self‐report data, and are only valid with normal or very mild cognitive decline in older adults (Lorette et al., 2021). Older adults are less likely to complain about sleep than younger adults, even if they have objective sleep disturbances (Gooneratne & Vitiello, 2014; Landry et al., 2015). Accordingly, reliance on subjective measures of sleep quality may prevent appropriate care‐seeking and biased results in scientific studies, especially in older adults. Therefore, objective sleep monitoring is essential, and subjective assessments should not be used as a substitute for objective sleep monitoring but as a complement to it (Landry et al., 2015). Objective monitoring of sleep is important to identify physiological parameters of sleep and their relationship with health as well as diagnosis of sleep‐related disorders (McCarter et al., 2022). The most commonly used method in clinical settings and the gold‐standard for diagnosing sleeping disorders is polysomnography (PSG), which tracks sleep stages and cycles to identify disruptions in sleep quality. During PSG, electrodes are placed on the scalp to simultaneously record cortical activity (electroencephalogram [EEG]), submental muscle activity (electromyogram [EMG]) and eye movements (electrooculographic activity [EOG]; Butkov & Keenan, 2017). However, PSG has several limitations, including being a one‐time assessment in a sleep lab, requiring expert interpretation of signals, and lacking the ability to capture long‐term and night‐to‐night variability of sleep patterns. Even if PSG is performed at home with home‐based ambulatory PSG, the limitations regarding the requirement of an expert for interpretation and intrusiveness remain the same (Ong et al., 2023). PSG is mainly used to diagnose sleep disorders rather than for long‐term sleep monitoring. Even with sleep disorders, night‐to‐night variability is important to accurately diagnose and monitor its severity (Lechat, Scott, et al., 2023). Furthermore, sleep‐monitoring devices for older adults should be user‐friendly and non‐intrusive, with consideration of their unique needs to enhance device acceptance and compliance (LeBlanc et al., 2022; Starr et al., 1939). Additionally, sleep in a laboratory setting may differ from sleep at home (Rundo & Downey, 2019). Home‐based sleep monitoring is shown to be associated with improved SE, longer sleep duration (SD), shorter SOL and increased time spent in REM sleep than lab‐based sleep monitoring (Bruyneel et al., 2011). There are several other objective sleep‐monitoring methods and tools that can be performed in the home environment without the complexities and limitations of PSG. Previous studies and reviews have effectively summarized the subjective measures and instruments to monitor sleep (Fabbri et al., 2021; Ji & Liu, 2016). There are also scoping reviews conducted on objective sleep monitoring. However, they are either not specifically targeted for older adults (Baron et al., 2018), or their research questions do not include a comprehensive classification of technologies with their sensors and objective sleep parameters and their associations with different types of predictors and outcomes (Kim et al., 2010). Therefore, the primary objective of this study is to provide a comprehensive overview of the current state of the art in home‐based objective sleep monitoring for older adults. This includes an examination of the technologies, sensors and sleep parameters utilized. Additionally, the study aims to explore various predictors and outcomes associated with sleep to gain a comprehensive understanding of home‐based sleep monitoring and its determinants.
2. RESEARCH METHODOLOGY
2.1. Overview
In this study, we chose to perform a scoping review based on the framework proposed by Arksey & O'Malley (2007). A scoping review is a suitable option for examining a broad range of literature on a particular topic in order to determine the scope and diversity of the research activity. Arksey & O'Malley (2007) proposed the methodology of a scoping review to consist of the following steps:
Identifying the research question/s
Identifying relevant studies
Study selection
Charting the data
Collating, summarizing and reporting the results
The PRISMA flow diagram for the literature search was used for a systematic and transparent research process.
2.2. Step 1: Research questions
The main research questions that were identified for this review were as follows.
What technologies and sensors are used to monitor objective sleep at home in older adults?
Which objective parameters of sleep are used to measure sleep in older adults?
Which predictors and outcomes are measured in the studies of objective sleep monitoring in older adults?
What predictors and outcomes are associated with the most common objective parameters of sleep in the studies of objective sleep monitoring in older adults?
2.3. Step 2: Searching and identifying relevant studies
We developed a search strategy by identifying keywords using the Population, Concept, Context (PCC) framework (Pollock et al., 2023). Similar terms were explored using Mesh (PubMed), SvenskMesh, thesaurus.com and keywords used in similar articles searched through Google Scholar. Initial search strings were formulated and piloted in the relevant databases. The search strings were further refined by consulting with the librarian at the authors' affiliated institution. A systematic search was conducted in Scopus, Web of Science and PubMed databases. Keywords for search are available in Appendix A in Figure A1. The date of the final search was 27 February 2023. The full electronic search strategy for Pubmed is shown in Appendix B in Figure B2. The records were exported from each database into a web tool, Rayyan (Ouzzani et al., 2016), where duplicates were removed, and articles were screened by title and abstract for eligibility. After the final retrieval of selected articles, an AI‐based search was done on the Research Rabbit (RR) (Sharma et al., 2022) as an added check to ensure no important articles are missed to be included. This added check helped us to identify 10 more research studies. After applying the inclusion and exclusion criteria on these studies, five of these were included in the list of primary studies for this scoping review.
2.4. Step 3: Study selection
To select the primary studies, three independent reviewers evaluated the initial set of retrieved research studies, and applied inclusion and exclusion criteria developed based on the research questions. The step‐by‐step application of the study selection process is shown in Figure 1.
FIGURE 1.
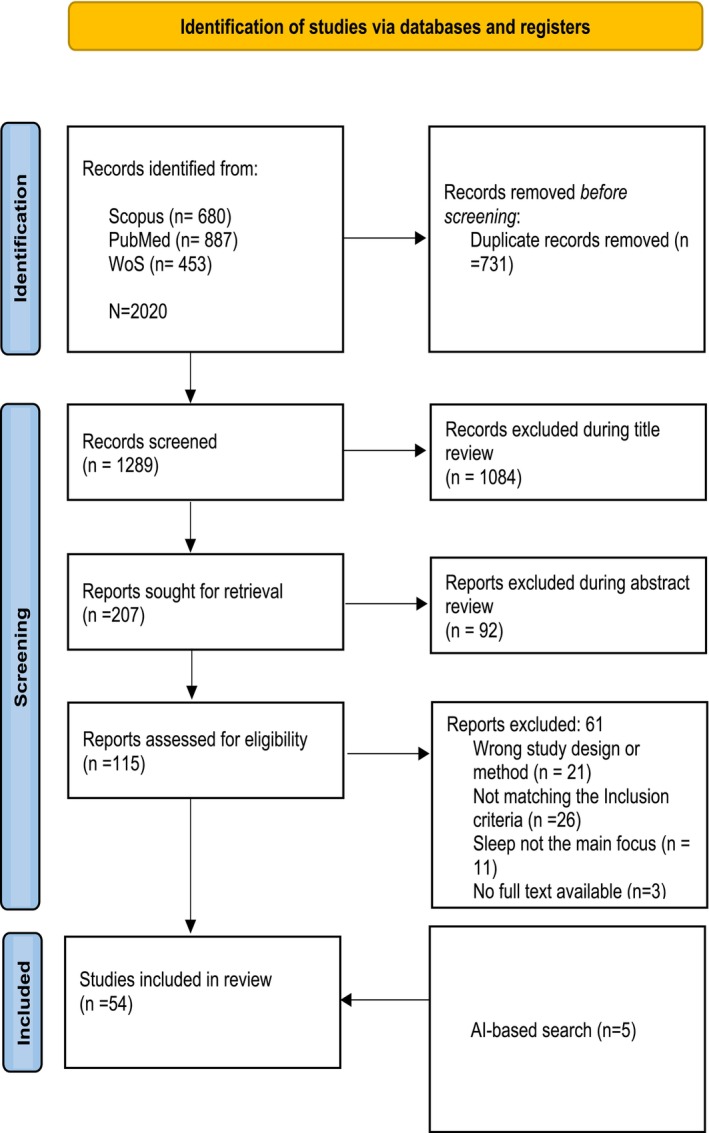
PRISMA‐ScR flow chart of study selection.
2.4.1. Inclusion criteria
The inclusion criteria for the scoping review were focused on studies that examined the use of wearable and non‐wearable devices for sleep monitoring in older adults, specifically those aged 60 years and above, in a home setting. The criteria were expanded to include studies where the majority of participants were aged 60 years or older. Eligible publications were required to have been published in English from 2008 to 2023, with the search concluding on 27 February 2023. The scope of included articles was limited to those focusing on sleep health or sleep quality, rather than sleep disturbances. Additionally, the studies were to emphasize objective sleep monitoring over technology specifications.
2.4.2. Exclusion criteria
In this scoping review, specific exclusion criteria were applied to refine the study's scope. Grey literature, systematic literature reviews (SLRs) and other forms of literature reviews were excluded. Additionally, studies that lacked age specifications for participants, used PSG as the sleep measurement tool, or were conducted outside a home setting were deemed ineligible. Moreover, studies not primarily focused on sleep parameters and their associations with various predictors and outcomes, or those that concentrated on technological specifications rather than sleep monitoring, were also excluded. The aim of these criteria is to ensure a focused analysis of the use of wearable and non‐wearable devices for sleep monitoring in older adults in a home setting.
2.5. Step 4: Charting the data
In this data extraction process, a data charting form was developed by identifying variables corresponding to the research questions. Data charting was done by the first author but, during the pilot run, the first three authors had discussions back and forth to finalize the template. Table C1 in Appendix C shows the charting of data done during this scoping review.
2.6. Step 5: Collating, summarizing and reporting the results
The collating and summarizing of the data was performed after the data charting. A general summarizing of the number of studies according to the location and year of publication was performed. The sample size was summarized from small to large, and a median was calculated. In addition to general summarizing, a specific collating and summarizing of the data was conducted in accordance with each research question. The technologies were categorized into wearables and non‐wearables, while sensors used in the studies were categorized into different categories. The objective parameters of sleep were listed, and each study was vigilantly read to understand the definition of each parameter. Thereafter, several different parameters were collated into one to avoid any redundancy. For example, SD or total time of sleeping was collated into TST. For the third question, the data were collated into four categories and summarized. Consequently, this led to the fourth question, where the most common objective markers of sleep were chosen and identified common predictors and outcomes associated with them. The results were then reported to present a comprehensive view. The descriptive numerical summary was presented to provide key quantitative information, such as the number of studies reviewed and the distribution of sleep parameters across various studies. In addition, common themes/categories were reported to highlight recurring patterns or trends, such as types of technologies, predictors and outcomes associated with sleep parameters in the studies. Finally, a data charting table was included to organize and report the extracted data from each study.
3. RESULTS AND ANALYSIS
3.1. General overview
Our search identified 2020 articles, of which 54 articles met the inclusion criteria. A PRISMA flow diagram of the scientific literature and selection criteria is presented in Figure 1. A total of 12 countries were represented in the studies. The two most common locations where the studies took place were the USA (n = 30) and Japan (n = 10). A total of three studies were conducted in Switzerland, two in Australia, and two in Canada, while all the other countries, as mentioned in Figure 2, had only one study each.
FIGURE 2.
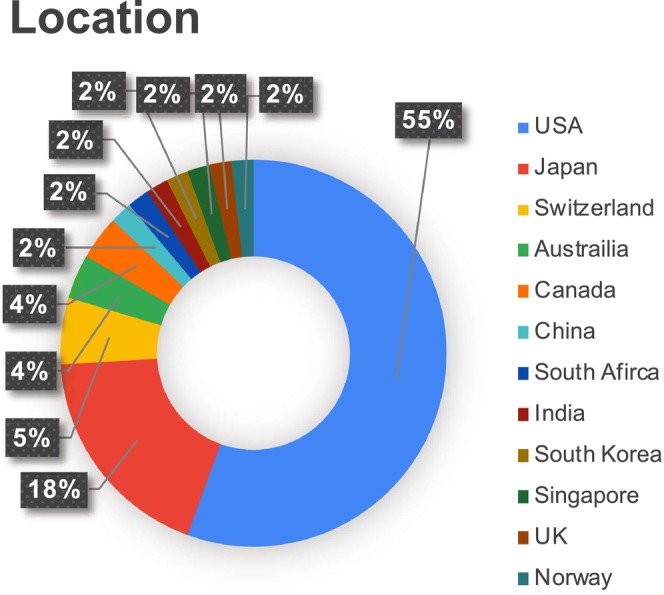
Percentage of studies based on locations.
This scoping review showed a fluctuating publication trend with a surge in publications around 2021 (n = 10). The graphical representation of this trend is shown in Figure 3. The sample size in the studies ranged from n = 8 (Tsuzuki et al., 2015) to n = 6107 (Patel et al., 2008), with a median of 103. In 35 studies, most of the sample population was female, and only one study had an equal number of both genders (Obayashi, Saeki, Iwamoto, et al., 2014). In most of the studies (n = 21), the majority of the population was Caucasians (white), and in only three studies, the majority of the population was African or African American (black). All the studies were quantitative because of the nature of the research questions. Fifty‐one studies were published in a medicine, health or public health journal.
FIGURE 3.
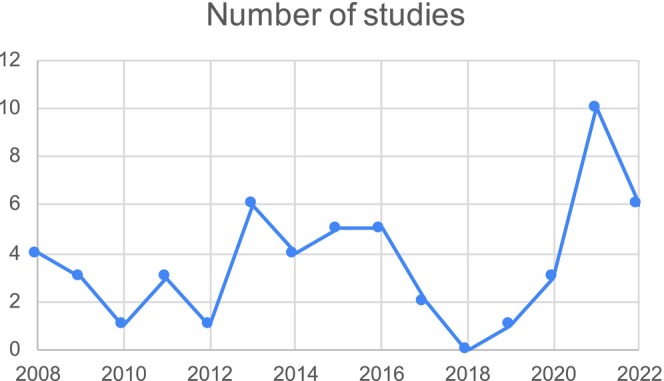
The number of studies over the years.
3.2. Technologies and sensors
All the technologies and sensors used to monitor sleep are mentioned in the data charting Table C1 in Appendix C. The data were collated into wearables and non‐wearables. Note that one of the studies used two devices to monitor sleep (Seelye et al., 2015). Among the 48 studies on wearables, 43 studies used wrist‐worn with actigraphy sensors, while three studies used headbands with EEG sensors (Altendahl et al., 2020; Lustenberger et al., 2022; Scullin, 2013) and two used actigraphy sensors around the waist/hip (Full et al., 2020; Miyazaki et al., 2021). Among the non‐wearable devices, three studies used near‐bed devices, including in‐home sensor networks using motion sensors (Seelye et al., 2015) and passive infrared sensors (Reynolds et al., 2022), as well as bedside monitors using radiofrequency sensors (Mahajan et al., 2021). Furthermore, four studies used under‐mattress mats to monitor sleep with ballistocardiography (BCG; Gao et al., 2021; Kholghi et al., 2021; Rawtaer et al., 2020; Schütz et al., 2021), while one study used a motion sensor under the mattress along with in‐home sensor networks using motion sensors. (Seelye et al., 2015). The study (Rawtaer et al., 2020) did not mention the brand name, except that it used fibre‐optic technology with BCG sensors placed under the mattress. All the sensors used are summarized in Figure 4, where the size of each bubble represents the number of studies in which the sensors are used. Most of the studies used manufacturers' software to analyse the raw data from the device (n = 39), while five studies did not mention how the analysis of raw data was conducted. Thirteen studies utilized algorithm‐based analysis or a combination of the manufacturer's software and algorithms to determine specific parameters like sleep onset and offset.
FIGURE 4.
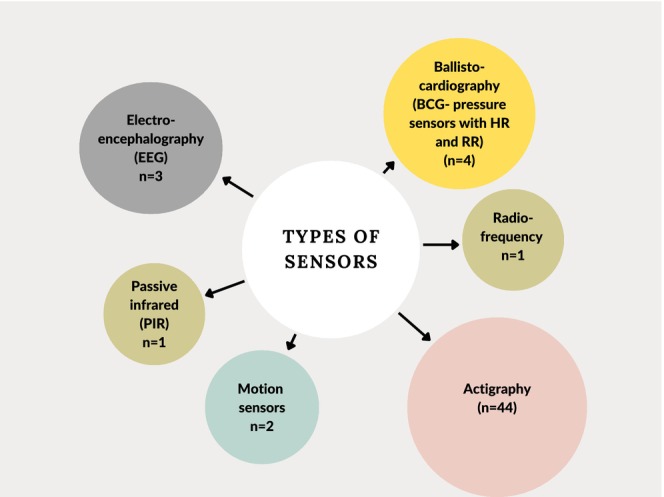
Types of sensors. The size of each bubble represents the number of studies in which the sensors are used. HR, heart rate; RR, respiratory rate. Note that one of the studies used two devices to track sleep, so the total here becomes 55 instead of 54 studies.
3.3. Objective parameters of sleep
Figure 5 shows all the objective parameters used in the studies to evaluate sleep. Our results show that the most common parameters used in the studies are TST (n = 44), WASO (n = 33) and SE (n = 32).
FIGURE 5.
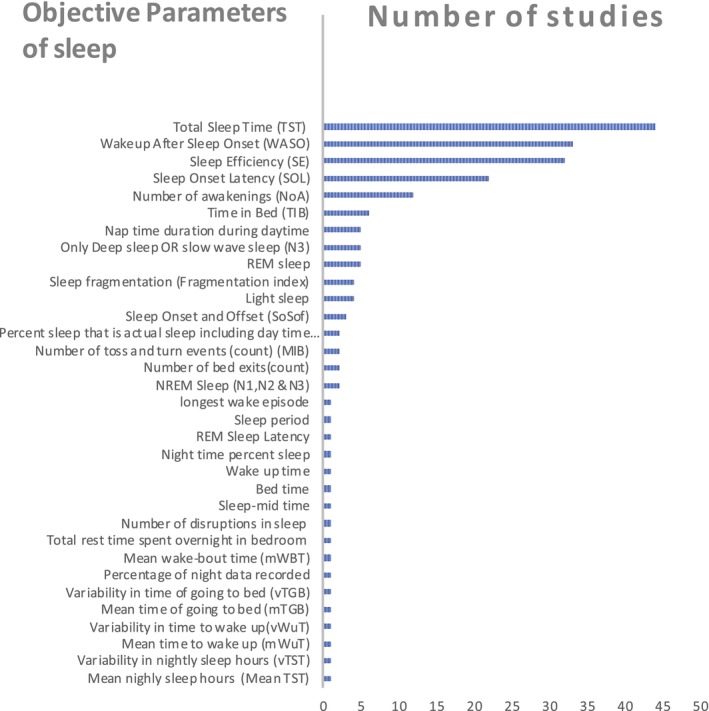
Objective parameters of sleep. NREM, non‐rapid eye movement; REM, rapid eye movement.
Six studies evaluated the sleep architecture with sleep stages (Altendahl et al., 2020; Lustenberger et al., 2022; Mahajan et al., 2021; Schütz et al., 2021; Scullin, 2013; Tadokoro et al., 2020). These parameters were obtained by using different kinds of sensors, for example, EEG (Altendahl et al., 2020; Lustenberger et al., 2022), BCG (Schütz et al., 2021), Radiofrequency (Mahajan et al., 2021) and Actigraphy sensors (Tadokoro et al., 2020).
Scullin (2013) used a headband device that measured only slow‐wave sleep. The studies (Altendahl et al., 2020; Lustenberger et al., 2022; Schütz et al., 2021) assessed non‐rapid eye movement (NREM) sleep, encompassing light‐stage sleep (Stages 1 and 2), deep‐stage sleep (slow‐wave sleep, Stage 3), and REM sleep, serving as objective markers of sleep stages. Another study (Tadokoro et al., 2020) assessed the sleep architecture with the same parameters as in studies (Altendahl et al., 2020; Lustenberger et al., 2022; Schütz et al., 2021) in addition to REM latency.
3.4. Types of predictors and outcomes measured in studies of objective sleep monitoring
The total number of associations measured in 54 studies was 75. To understand the overall landscape of outcomes that are studied with sleep parameters, we collated the outcomes in different categories, as mentioned in Table 1.
TABLE 1.
Predictors and outcomes analysed in the studies against sleep.
| Predictors and outcomes category | Number of related associations |
|---|---|
| Health‐related | 37 |
| Environmental | 16 |
| Interventional | 9 |
| Behavioural | 7 |
| Time and place | 4 |
| Social | 2 |
Health‐related predictors and outcomes include human immunodeficiency virus (HIV) status (Redman et al., 2022), cognitive performance (Bernstein et al., 2022), autonomic regulation through heart rate variability (Huang et al., 2022), daily functioning (Lustenberger et al., 2022; Parsey et al., 2015), peritoneal dialyses specific factors like haemoglobin and phosphorous level (Zhang et al., 2021), mild cognitive impairment (MCI; Gao et al., 2021; Rawtaer et al., 2020), Alzheimer's disease (Gao et al., 2021; Lim et al., 2013; Wams et al., 2017), perceived health status (Schütz et al., 2021), markers of cardiometabolic health (Full et al., 2020), neural structure, i.e. white matter of the brain (Altendahl et al., 2020), daytime cortisol level (Morgan et al., 2017), amnesic MCI (aMCI; Wams et al., 2017), inflammatory burden (Smagula et al., 2016), APOE e4 (a risk factor allele for Alzheimer's disease; Drogos et al., 2016), peripheral arterial disease (Nagayoshi et al., 2017), working memory (Miyata et al., 2013; Seelye et al., 2015), number of falls (Stone et al., 2014), obesity (Patel et al., 2014, 2008), episodic memory (Scullin, 2013), sustained attention and vigilance (Miyata et al., 2013), instrumental activities of daily living (IADL) impairment (Spira et al., 2012), grip strength (Spira et al., 2012), gait speed (Spira et al., 2012), subjective pain (Dzierzewski et al., 2010), frailty status (Ensrud et al., 2009), anxiety (Spira et al., 2009), testosterone levels (Barrett‐Connor et al., 2008), cognitive decline (Blackwell et al., 2014; Lim et al., 2013) and depression (Maglione et al., 2008; Paudel et al., 2009).
Environmental predictors and outcomes that were measured in the studies for association with sleep parameters are seasonal variations in patients with MCI (Reynolds et al., 2022), perceived household environment (Johnson et al., 2021), interior housing conditions (Okoye et al., 2021), exterior housing conditions (Okoye et al., 2021) neighbourhood disorder (Okoye et al., 2021), evening indoor temperature (Saeki et al., 2015), initial bed temperature (Okamoto‐Mizuno & Tsuzuki, 2010; Saeki et al., 2015), seasonal thermal environment (Okamoto‐Mizuno & Tsuzuki, 2010; Tsuzuki et al., 2015), illuminance (Obayashi, Saeki, & Kurumatani, 2014; Tsuzuki et al., 2015), evening light exposure (Obayashi, Saeki, Iwamoto, et al., 2014), daytime‐light exposure (McCurry et al., 2011), at‐night skin temperature (Okamoto‐Mizuno & Tsuzuki, 2010) and relative humidity (Okamoto‐Mizuno & Tsuzuki, 2010).
Interventional predictors and outcomes are few that involved intraocular lens placement in cataract patients (Chellappa et al., 2022), auditory deep stimulation (Lustenberger et al., 2022), interval training with sleep hygiene (Mahajan et al., 2021), 3‐month light to moderate intensity aerobic exercise (Miyazaki et al., 2021), CogniFit intervention (a cognitive training program; Haimov & Shatil, 2013), yoga intervention (Taibi & Vitiello, 2011), guided sleep education (NITE‐D; McCurry et al., 2011), sleep hygiene program with bright and dim light exposure in morning or evening (Friedman et al., 2009), and daytime light exposure (Martin et al., 2008).
Behavioural predictors and outcomes consisted of mobility in terms of daily steps (Gao et al., 2021), behavioural and psychological symptoms of dementia (Cho et al., 2021), in‐bed behaviours (Johnson et al., 2021), number of baths in 3 days (Tai et al., 2021), bathing duration (Tai et al., 2021), time interval between bathing and bedtime (Tai et al., 2021), and walking (McCurry et al., 2011).
Predictors and outcomes regarding time and place are measured by comparing sleep between two different times or places. These include sleep measures pre‐ and post‐lockdown (Kholghi et al., 2021), nursing home residents versus community‐dwelling residents (Kume et al., 2016; Olsen et al., 2016), and sleep in assisted living facilities versus sleep at home (Martin et al., 2008). Social predictors and outcomes are measured only in one study where partnership status and relationship quality are assessed in association with sleep parameters (Chen et al., 2015).
3.5. Predictors and outcomes associated with objective parameters of sleep
In our 54 studies, we found that several objective parameters of sleep were associated with several diverse predictors and outcomes mentioned in the previous section. Table 2 shows the predictors and outcomes that were associated with TST, WASO and SE. TST was associated with 14 health‐related, four environmental, two interventions, three behavioural, two time and place, and one social predictors and outcomes. WASO was associated with nine health‐related, four environmental, four interventions, three behavioural, one time and place, and one social predictors and outcomes. SE was associated with 10 health‐related, four environmental, two interventions, two behavioural, one time and place, and one social predictors and outcomes.
TABLE 2.
Association of objective parameters of sleep with health, environmental, interventions, behavioural, time and place, and social predictors and outcomes.
| Predictors and outcomes | TST | WASO | SE |
|---|---|---|---|
| Health‐related | HIV status, HR variability, effectiveness of peritoneal dialysis (Hb, Ph levels), markers of cardiometabolic health, aMCI, inflammatory burden, peripheral arterial disease, number of falls, obesity, working memory capacity, IADL impairment, grip strength, subjective pain, frailty status | HR variability, daytime cortisol levels, inflammatory burden, grip strength, frailty status, anxiety, depression, testosterone levels, cognitive functional decline | Effectiveness of peritoneal dialysis (Hb, Ph levels), APOE e4, number of falls, working memory capacity, IADL impairment, grip strength, frailty status, anxiety, testosterone levels, cognitive functional decline |
| Environmental predictors and outcomes | Exterior housing conditions, seasonal thermal environment, illuminance, outside and inside temperature | Exterior housing conditions, seasonal thermal environment, illuminance, day‐time light exposure | Exterior housing conditions, seasonal thermal environment, illuminance, outside and inside temperature |
| Interventions | 3‐month light to moderate intensity aerobic exercise, sleep hygiene and exposure to bright light | Interval training with sleep hygiene, 3‐month light to moderate intensity aerobic exercise, CogniFit intervention, guided sleep education; NITE‐D | 3‐month light to moderate intensity aerobic exercise, CogniFit intervention |
| Behavioural | Mobility, BPSD, in‐bed behaviours | Mobility, in‐bed behaviours, walking | Mobility, in‐bed behaviours |
| Time and place | COVID‐19 pre‐ and post‐lockdown, living place | Living place | Living place |
| Social | Partnership status | Partnership status | Partnership status |
aMCI, amnestic mild cognitive impairment; BPSD, behavioural and psychological symptoms of dementia; Hb, haemoglobin; HIV, human immunodeficiency virus; HR, heart rate; IADL, instrumental activities of daily living; Ph, phosphorous; SE, sleep efficiency; TST, total sleep time; WASO, wake after sleep onset.
The 10 common predictors and outcomes associated with all three most common parameters are shown in Figure 6. Two of these were health‐related, frailty (Ensrud et al., 2009) and grip strength (Spira et al., 2012); three were environmental, which included exterior housing conditions (Okoye et al., 2021), seasonal thermal environment (Okamoto‐Mizuno & Tsuzuki, 2010; Tsuzuki et al., 2015) and illuminance (Obayashi, Saeki, & Kurumatani, 2014; Tsuzuki et al., 2015); two were behavioural associations, like in‐bed behaviours (Johnson et al., 2021) and mobility (Gao et al., 2021). One intervention, 3‐month light to moderate intensity aerobic exercise intervention (Miyazaki et al., 2021), living place, i.e. nursing homes versus community‐dwelling homes (Kume et al., 2016; Olsen et al., 2016), and a social outcome of partnership status (Chen et al., 2015) were associated with TST, WASO and SE. The objective parameters with sleep stages are associated with auditory deep sleep stimulation (Lustenberger et al., 2022), daily functioning (Lustenberger et al., 2022), Alzheimer's disease (Tadokoro et al., 2020), MCI (Tadokoro et al., 2020), neural structure, i.e. the white matter of the brain (Altendahl et al., 2020), and episodic memory (Scullin, 2013).
FIGURE 6.
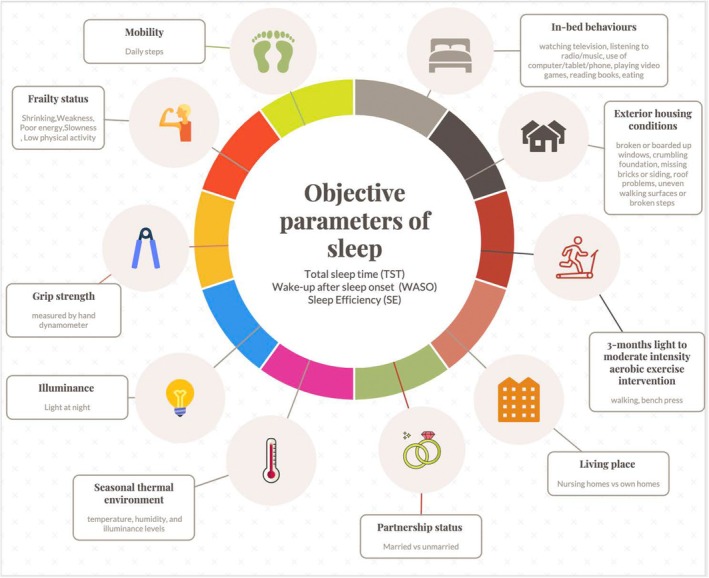
The 10 predictors and outcomes that are associated with the most common objective parameters of sleep i.e. TST, WASO and SE. The figure illustrates that TST, WASO and SE are associated with, for example, grip strength, partnership status, frailty status, etc. SE, sleep efficiency; TST, total sleep time; WASO, wake after sleep onset.
4. DISCUSSION
Key findings
The predominant method for home‐based objective sleep monitoring in older adults involved using a wearable, wrist‐worn device with actigraphy sensors.
Among the various objective parameters of sleep, TST, WASO and SE were the most frequently measured parameters in older adults. In the limited number of studies that measured sleep architecture, the parameters of sleep commonly used were slow‐wave sleep (N3 or deep sleep) and REM sleep.
Predictors and outcomes assessed in studies of objective sleep monitoring in older adults were health‐related, environmental conditions, interventions, behavioural aspects, time and place considerations, and social associations.
In older adults, sleep parameters like TST, WASO and SE were associated with in‐bed behaviours, exterior housing conditions, 3‐month light to moderate intensity aerobic exercise, living place, relationship status, seasonal thermal environment, illuminance, grip strength, frailty status and mobility. Specific sleep stages were associated with auditory deep sleep stimulation, daily functioning, Alzheimer's disease, MCI, neural structure and episodic memory.
Our findings provide knowledge of the overview of home‐based objective sleep monitoring in older adults. The majority of studies on objective sleep monitoring at home in older adults were conducted in the USA. Among the European states, only Switzerland, the UK and Norway were represented, indicating a research gap in these regions. In recent years, especially during and after 2021, there has been an increase in studies focusing on home‐based objective sleep monitoring in older adults. The need for telehealth solutions and home‐based monitoring systems rose with the implementation of social distancing measures and the trend towards remote service. This change was necessary to maintaining healthcare delivery while lowering the risk of viral transmission, and it increased interest in creating and evaluating new technology that could facilitate these adjustments (Warrier & Sood, 2021). These immediate adaptations within healthcare research during the pandemic might be the reason for the reflected surge of research articles during 2021.
The review pointed out that actigraphy is the most used in‐home sleep‐monitoring method for older adults. The actigraphy technology collects data generated by movement or activity, and is either a wrist‐worn or hip/waist‐worn device. Although actigraphy is convenient and comfortable, a study shows that sleep parameters derived from actigraphy are less accurate than EEG (Rocknathan et al., 2017). In a study, it was found that actigraphy tends to overestimate light sleep bias more in the older age group than in the younger age group (Ong et al., 2023). EEG sensors monitor changes in brain activity, which in our review was performed through a headband device. The headband devices are comfortable and patient‐friendly, have a high level of accuracy, and provide accurate physiological signals (Arnal et al., 2020). Compared with the gold‐standard PSG, EEG headbands provided the lowest discrepancies in sleep parameters among other sleep monitors. These headbands are also recommended for evaluating poor sleep quality or when the highest degree of accuracy in sleep staging is required (Ong et al., 2023). There are other technologies than headbands that use EEG for sleep monitoring, such as ear‐EEG sleep‐monitoring plugs and patches (Mohamed et al., 2023). Our scoping review revealed a research gap in the use of these devices for older adults in their home environment. Another method of sleep monitoring we found in our studies is BCG. A BCG measures “the recoil force of the body in reaction to cardiac ejection of blood into the aorta and provides information on the timing of each heartbeat” (Starr et al., 1939). All of the BCG sensors, according to our review, are installed as under‐mattress mats. The use of these devices has the potential to empower individuals to improve their sleep quality. On one hand, the use of screens and connectivity is generally perceived as disruptive to sleep. On the other hand, novel digital technologies could potentially facilitate sleep health and aid in the identification of disruptions of sleep parameters and sleep disorders (Jaiswal et al., 2019). A further review study is required that investigates the user‐friendliness, non‐intrusiveness, acceptance and compliance of these technologies in older adults (LeBlanc et al., 2022; Sadek & Mohktari, 2018).
The objective parameters that were most commonly found in our study were TST, WASO and SE. Sleep quality is measured using various technologies that assess different aspects of sleep. These technologies generate diverse parameters used to determine the objective parameters of sleep, such as TST, WASO and SE. TST refers to the total duration of sleep, WASO measures the total amount of time a person spends awake after falling asleep and is measured in minutes, and SE is the percentage of time spent asleep while in bed (O'Donnell et al., 2009). The literature supports that TST decreases with age from childhood to late adulthood, but then plateaus after 60 years old (Li et al., 2022). WASO, which is a measure of sleep maintenance, also worsens as aging occurs but remains unchanged after 60 years (Ohayon et al., 2004). SE remains consistent from childhood to adolescence and declines significantly with age in adulthood, and it declines very slowly further on with advancing age (Ohayon et al., 2004). There is significant night‐to‐night variability in objective sleep parameters in older adults (McCarter et al., 2022; Parsey et al., 2015). Even among those averaging the recommended 7–9 hr of sleep, 40% of nights fell outside this range (Scott et al., 2024). Daily monitoring of sleep parameters can be a powerful tool for older adults and their healthcare providers. It can help identify potential sleep‐related disorders (Lechat, Scott, et al., 2023) at an early stage, and health factors that affect the variability in these parameters (Lechat, Loffler, et al., 2023). Single‐night parameters may result in misdiagnosis and undertreatment of patients with mild sleep disorder symptoms during the monitoring period (Lechat et al., 2022). Therefore, these sleep parameters, through daily monitoring, provide a more comprehensive and accurate picture of an individual's sleep health.
There were several predictors and outcomes that were found to be associated with the most common objective parameters of sleep in older adults. They were health‐related, environmental, interventions, behavioural, time and place, and social. Home‐based sleep monitoring can be used to explore the link between sleep and various factors by measuring night‐to‐night sleep variability and objective measures of sleep across multiple nights. Analysis of sleep parameters and their association with multidimensional associations reveals a complex interplay that impacts healthy aging. Health‐related associations such as frailty and grip strength are associated with TST, SE and WASO. Grip strength is often used as a measure of overall physical health and functional ability, while frailty, which encompasses physical, cognitive and social elements, has also been linked to reduced grip strength (Pana et al., 2021). Sleep quality and grip strength have a bidirectional association, with sleep quality affecting grip strength and vice versa (Han et al., 2023). The interconnectedness of objective sleep, frailty and grip strength highlights the importance of sleep in the overall wellbeing of older adults (Ravyts & Dzierzewski, 2022). Environmental associations such as exterior housing conditions, seasonal thermal environment, and illuminance are also found to be associated with sleep parameters TST, SE and WASO. Several environmental factors, both indoors and outdoors, play an important role in sleep quality. Exterior housing conditions, such as poorer housing quality, were found to be associated with shorter SD (Troxel et al., 2020). Being exposed to continuous ambient noise levels of 39 dBA or higher is connected to more frequent awakenings at night, shorter duration of sleep, diminished sleep quality, and a decrease in REM sleep (Marks & Griefahn, 2007). Light at night affects the sleep quality through increased awakenings and reduced slow‐wave sleep (Cho et al., 2013). By facilitating an environment conducive to healthy sleep, public health workers can contribute to better sleep for older adults. Apart from environmental associations, in‐bed behaviours such as watching TV or using screens have been found to be associated with TST, WASO and SE. A recent review highlighted that the relationship between technology use and sleep may be bidirectional. Technology use can affect sleep by displacing bedtime and delaying sleep onset, while sleep problems may lead to increased technology use as a way to fill time, provide cognitive distraction or aid in emotional regulation (Bauducco et al., 2024). These results about the association of objective parameters and sleep in older adults indicate that different behaviours, the environment, and the time before and after the pandemic can affect brain activity and, thus, inevitably affect sleep. Objective sleep monitoring, simultaneously with other technologies that help in relaxation and maintaining sleep hygiene, can be useful in improving sleep quality (Leonidis et al., 2021). The result of the analysis provides valuable insights to guide the development of future research. They can guide interventions to address key factors that significantly impact the quality of sleep among older adults. For example, interventions of changing in‐bed behaviours or exterior environmental conditions may improve the SD, SE and sleep maintenance of older adults (Koch et al., 2006). Recognizing the associations between specific sleep stages and conditions such as Alzheimer's disease, MCI, neural structure and episodic memory can have significant implications for cognitive health. Addressing sleep disturbances in older adults could help maintain cognitive function and potentially lower the risk of cognitive decline (Nyholm et al., 2024).
There has been limited research on daily monitoring of sleep stages in older adults in home environments. Monitoring sleep stages, such as REM sleep and NREM sleep (N1, N2 and N3 or deep sleep), provides detailed insight into the quality and depth of your sleep (McCarter et al., 2022). The disorders in specific stages of sleep might be an indicator of a potential health condition. For example, increased time in stage N1 sleep and less time in stage REM sleep are associated with worsening cognitive performance in older men over time (Song et al., 2015). Disturbances in NREM sleep are associated with the development of Alzheimer's disease. This is due to the insufficient functioning of the brain's glymphatic system, leading to the accumulation of amyloid beta, a hallmark of Alzheimer's disease (Astara et al., 2023). Therefore, distinguishing sleep stages is important in older adults to monitor and diagnose sleep disturbances and health outcomes. Non‐EEG‐based technologies are currently not accurate enough to reliably distinguish sleep stages when compared with the gold‐standard of PSG (Imtiaz, 2021; Yuan et al., 2024). Recent advances in wearables using EEG technology have shown high accuracy, especially in sleep staging, supported by feasibility studies and validation across populations. These devices offer the potential for more accurate at‐home sleep monitoring, with high compliance and user‐friendliness (de et al., 2024).
There are several strengths of this review. This review entailed a comprehensive search, which was reviewed and improved with the help of a librarian. The meticulous following of the methodology led to a detailed extraction process, which was discussed on a number of occasions among the three authors. To our knowledge, this is the first scoping review regarding home‐based objective sleep monitoring at home in older adults, and its associations with diverse predictors and outcomes.
There are some limitations of our study that are also important to consider. Our search of the articles was limited to English, which may have led to the exclusion of important studies. The exclusion of the articles after 27 February 2023 and articles that published algorithms based on machine learning or described technologies without exploring the predictors and outcomes related to sleep parameters might have led to leaving out recent technologies. Furthermore, the exclusion of studies focused on sleep disturbances and clinical sleep disorders also limits the applicability of the findings to populations with sleep disorders. One limitation of this review is the inability to provide validation of the technologies provided, as this was out of the scope of the review. Future studies should comprehensively study validations, and disclose funding and conflicts of interest to enhance clarity and depth of information.
5. CONCLUSIONS
This scoping review adds to the expanding body of knowledge on objective sleep monitoring at home in older adults, highlighting various predictors and outcomes associated with sleep parameters. In summary, the predominant method for home‐based objective sleep monitoring involves using a wearable, wrist‐worn device with actigraphy sensors. Among the various objective parameters of sleep, TST, WASO and SE are the most frequently measured parameters in older adults. In older adults, sleep parameters like TST, WASO and SE are associated with in‐bed behaviours, exterior housing conditions, 3‐month light to moderate intensity aerobic exercise, living place, relationship status, seasonal thermal environment, illuminance, grip strength, frailty status, and mobility. The results of our study hold significant implications for tailored interventions, environmental modifications, early detection of sleep irregularities, and a comprehensive understanding of the interplay between sleep and the many factors that impact the aging population. The gaps identified, particularly exploring sleep architecture using home‐based objective sleep monitoring in older adults, offer a roadmap for future research.
FUNDING INFORMATION
This study has not received any external funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflict of interest.
Supporting information
TABLE S1. Charting of data with ethnic background, gender and publishing venue.
ACKNOWLEDGEMENTS
The authors thank the Swedish National Graduate School on Aging and Health for supporting the learning process. The authors would also like to thank Josefin Andersson and Ottilia Sundahl, the librarians at Blekinge Institute of Technology, for their help in refining the search strings.
APPENDIX A.
KEYWORDS FOR SEARCH
FIGURE A1.

Search keywords.
APPENDIX B.
EXAMPLE OF SEARCH STRING IN PUBMED
FIGURE B2.

Search string example from PubMed.
APPENDIX C.
DATA CHARTING
TABLE C1.
Data charting Table‐List of studies included in the review.
| Article | Year | Sample size | Location | Sample age in years | Type of monitoring technology | Sensors | Data analysis technique | Objective parameters of sleep | Predictors/outcomes | Parameters associated with predictors and outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. (Redman et al., 2022) | 2022 | 172 | South Africa | 45–93 (66.7 ± 11.5) | ActTrust. Condor instruments (wrist‐worn) | Actigraphy | Manually marked: sleep onset and offset, automated algorithm, Cole/Kripke algorithm in ActStudio | Sleep onset and offset, WASO, TST, SE (% time spent asleep between sleep onset and offset) | HIV status | Sleep onset and offset and TST |
| 2. (Bernstein et al., 2022) | 2022 | 91 | USA | 73.7 ± 5.7 | Withings steel (wrist‐worn) | Actigraphy | Not mentioned | Mean nightly sleep hours, variability in nightly sleep hours, mean time to wake up, variability in time to wake up, mean time of going to bed, variability in time of going to bed, and percentage of night data recorded | Cognitive performance |
Percentage of night data recorded Greater variability in wake time |
| 3. (Huang et al., 2022) | 2022 | 68 | USA | 69 ± 2 |
Actiwatch SpectrumPro, Phillips Respironics (wrist‐worn) |
Actigraphy | Actiwatch software (version 6.0) | TST, SE, WASO | Heart rate variability (autonomic regulation) | TST, WASO |
| 4. (Chellappa et al., 2022) | 2022 | 29 | Switzerland | 55–80 (69.8 ± 6.2 experiment, 63.6 ± 5.6) | Acti‐watch‐L, Cambridge Neurotechnology (wrist‐worn) | Actigraphy | Not mentioned | TIB, TST, SE, mean wake about time | Intraocular lens placement with previous cataract | None |
| 5. (Reynolds et al., 2022) | 2022 | 128 | USA | ≥ 60 (85.2 ± 7.7) | In‐home passive monitoring technology (NYCE, Vancouver, BC) | Infrared presence sensors | An algorithm was written in MATLAB to estimate total rest time from the firing timestamps of the bedroom sensors and sensors in neighbouring rooms | Total rest time in bedroom (TRTiB) |
MCI older adults seasonal variation |
TRTiB |
| 6. (Lustenberger et al., 2022) | 2022 | 16 | Switzerland | 62–78 (69.5 ± 1.3) | MHSL‐SleepBandv2 (Headband) | EEG, EMG, EOG | Algorithm was written in MATLAB to classify sleep as NREM and REM | NREM sleep (sleep stages N2 and N3 characterized by SW and sleep spindles) or not‐NREM sleep (awake, N1 and REM) | Auditory deep sleep stimulation and daily functioning | Slow‐wave activity (NREM) |
| 7. (Gao et al., 2021) | 2021 | 36 | Australia | ≥ 65 (83.5 ± 7.0) | EMFIT QS sensor (mattress‐based) | BCG | Not mentioned | TST, SOL, WASO, TIB, NoA, SE | Mobility (daily step) | SE, WASO, TST |
| 8. (Mahajan et al., 2021) | 2021 | 13 | India | ≥ 60 (74.16 ± 6.17) | S+ Sleep monitor (manu. by ResMed.) (bedside, connected to a smartphone through Bluetooth) | Directional radiofrequency sensor that collects data based on respiratory patterns of the participant during sleep | ResMed app for android v 1.2.0 (ResMed San Diego, CA) | Light sleep, deep sleep, REM sleep, wake time (WASO), TST, number of disruptions | Interval training with sleep hygiene | Wake time in minutes (WASO) |
| 9. (Cho et al., 2021) | 2021 | 204 | South Korea | ≥ 65 (81.23 ± 6.01) with dementia | wGT3X‐BT activity monitor; ActiGraph, LLC (wrist‐worn) | Actigraphy | ActiLife software (version 6.13.3; ActiGraph, LLC) Cole–Kripke algorithm to score a 1‐min epoch as asleep or awake | TST, WASO | Behavioural and psychological symptoms of dementia | TST |
| 10. (Zhang et al., 2021) | 2021 | 50 | China | ≥ 60 (70.4 ± 7.2) with end‐stage renal disease undergoing peritoneal dialysis |
ActiGraph GT3X, Pensacola, FL, USA (wrist‐worn) |
Actigraphy | ActiLife V6.13.3 software, wear time through Choi algorithm, sleep parameters through Cole–Kripke algorithm | TST, SE | Haemoglobin levels, serum phosphorus levels (disease peritoneal dialysis specific factor) | TST, SE |
| 11. (Johnson et al., 2021) | 2021 | 231 | USA | 66.3 ± 10.8 |
ActiGraph GT3X, Pensacola, FL, USA (wrist‐worn) |
Actigraphy | ActiLife V6.13.3 software use Cole–Kripke algorithm | TST, SE, WASO | a. Perceived household environment; b. in‐bed behaviours | a. No associated marker; b. TST, SE, WASO |
| 12. (Kholghi et al., 2021) | 2021 | 31 | Australia | ≥ 65 (846.8) | EMFIT QS sensor (mattress‐based) | BCG | Cloud‐based analysis platform | TST, SOL, WASO, SE | Sleep measures pre‐ and during lockdown | TST in women |
| 13. (Okoye et al., 2021) | 2021 | 136 | USA | ≥ 65 (76 ± 0) |
Actiwatch‐2 wrist actigraph (Philips Respironics, Bend, OR) (wrist‐worn) |
Actigraphy | Actiware Software 6.0.9 | TST, WASO, SE | a. Interior housing conditions; b. exterior housing conditions; c. neighbourhood disorder | a. Not statistically significant association; b. TST, WASO, SE; c. not statistically significant association |
| 14. (Tai et al., 2021) | 2021 | 1094 | Japan | ≥ 60 (72 ± 7.1) | Actiwatch‐2 wrist actigraph (Respironics, Murrysville, PA) (wrist‐worn) | Actigraphy | Actiware Software 5.5 Respironics | SOL | Day of bath (both, 1st day, 2nd day, neither), bathing duration, time interval between bathing and bedtime | SOL |
| 15. (Miyazaki et al., 2021) | 2021 | 49 | Japan | 65.7 ± 5.7 | Lifecorder GS (LC, Suzuken, Nagoya, Japan) (worn on hip) | Actigraphy | Sleep‐SignAct software program (version 2.0, Kissei Comtec, Matsumoto, Japan) with a scoring algorithm | TST, wake episode (NoA), WASO, SE, TIB | 3‐month light‐to‐moderate intensity aerobic exercise intervention | TST, WASO, SE, NoA |
| 16. (Rawtaer et al., 2020) | 2020 | 49 | Singapore | ≥ 65 (HC: 73 ± 5.3; MCI 75.1 ± 6.3) | Fibre‐optic technology placed under participants mattress | BCG | Not mentioned | SD (TST) interruptions | Sleep interruptions in MCI compared with healthy controls | Sleep interruptions were associated with MCI |
| 17. (Tadokoro et al., 2020) | 2020 | 63 | Japan | NC: 74.0 ± 7.8; MCI: 77.6 ± 6.5; AD: 75.5 ± 6.2 | WatchPAT (Itamar Medical, Caesarea, Israel) | Actigraphy | Algorithms | TST, REM, light sleep, deep sleep, SOL, REM sleep latency | Normal cognition, AD, MCI | REM, NREM |
| 18. (Schütz et al., 2021) | 2021 | 37 | Switzerland | 70–101 (87 ± 7) |
EMFIT QS, Emfit (mattress‐based) |
Respiration, heart rate | Proprietary algorithms from the device manufacturer | Number bed exits (count), number toss‐and‐turn events (larger movements, count), TST REM, light sleep, deep sleep, duration awake (WASO), SE, SOL | Health status EQVAS (equation 5D 3L) | Number of toss‐and‐turn events (larger movements, count) |
| 19. (Full et al., 2020) | 2019 | 3329 women | USA | 63–99 (78.5 ± 6) | ActiGraph GT3X+; Pensacola, FL (worn on the waist) | Actigraphy | ActiLife version 6.11 | TST | Markers of cardiometabolic health | TST |
| 20. (Altendahl et al., 2020) | 2020 | 43 | USA | 66–84 (75 ± 4.51) |
Zeo (headband) |
EEG | Proprietary algorithms from the device manufacturer | TST, SE, light sleep (LS) (stages 1 and 2 NREM sleep), deep sleep (DS) (Stage 3 NREM sleep) and REM sleep (RS) | White matter microstructure through DTI using fractional anisotropy & mean diffusivity | REM SLEEP |
| 21. (Morgan et al., 2017) | 2017 | 672 | USA | 66–78 (71.5 ± 0.0) | Actiwatch spectrum model, Philips, Respironics | Actigraphy | Actiware Software 5.5 Respironics | TST, sleep fragmentation score, and WASO | Daytime cortisol level | Sleep fragmentation score, WASO |
| 22. (Wams et al., 2017) | 2017 | 24 | United Kingdom | 77.1 ± 4.0 | Actiwatch 7, CamNTech (wrist‐worn) | Actigraphy | Actiwatch activity and Sleep analysis (version 7, CamNTech) | Number of wake bouts, fragmentation index, TIB, sleep period, TST, SE, sleep start, sleep end | Sleep in healthy versus aMCI (AD patients) | TST, TIB, sleep period, sleep start |
| 23. (Kume et al., 2016) | 2016 | 17 | Japan | 82.2 ± 4.2 |
Actiwatch 2; Philips Respironics, USA (wrist‐worn) |
Actigraphy | Actiware System, version 6.08 (Philips Respironics, USA) | TST, SOL, SE, awakening time, awakening frequency | Nursing home residents with or without dementia versus community‐dwelling without dementia | SE, awakening time, awakening frequency |
| 24. (Olsen et al., 2016) | 2016 | 115 (dementia) | Norway | 82.6 ± 6.84 | ActiSleep+, ActiGraph, Pensacola, USA (wrist‐worn) | Actigraphy | ActiLife, software Version 6.11.2 (ActiGraph, Pensacola, USA) | TST, WASO, NoA, SE | Differences in sleep parameters in home‐dwelling persons and nursing home residents with dementia | Nursing home residents have lower TST, WASO and sleep during the night time than community dwelling individuals |
| 25. (Smagula et al., 2016) | 2016 | 2531men | USA | 75.3 ± 5.01 | The Octagonal Sleep Watch actigraphy, or SleepWatch‐O, (Ambulatory Monitoring, Ardsley, NY) (wrist‐worn) | Actigraphy | ActionW‐2 software (Ambulatory Monitoring, Ardsley, NY) | TST, SL, WASO | Inflammatory burden | TST, WASO |
| 26. (Drogos et al., 2016) | 2016 | 35 | Canada | ≥ 55 (64.9 ± 5.1) | AW‐2 Actiwatch (Minimitter; Philips Respironics, Murrysville, PA) (wrist‐worn) | Actigraphy | Actiware program (Philips Actiware 6.0.4) | TST, NoA, WASO, SE, SOL | APOE e4 (risk factor allele for AD) | SE |
| 27. (Nagayoshi et al., 2017) | 2016 | 650 | USA | (63 ± 0.00) | Actiwatch Spectrum (Philips Respironics, Murrysville, PA) (wrist‐worn) | Actigraphy | Actiware‐Sleep version 5.59 software | SD (TST) | Peripheral arterial disease | TST |
| 28. (Chen et al., 2015) | 2015 | 727 | USA | 62–90 (71.79) |
Actiwatch (Philips/Respironics 2010) (wrist‐worn) |
Actigraphy | Actiware software version 5.59 (Philips/Respironics 2010) | TST, percent sleep, sleep fragmentation index, WASO | a. Partnership status (married or unmarried); b. relationship quality | a. TST, WASO; b. no association |
| 29. (Seelye et al., 2015) | 2015 | 63 | USA | 87.2 ± 6.2 | Home sensor network in the bedroom and pressure mats under beds (BAM Labs, Los Gatos, CA) | Passive motion sensor | Self‐created algorithms | Total movement in bed at night (MIB), times up at night, and TST | Neuropsychological performance (working memory) | Mean MIB |
| 30. (Saeki et al., 2015) | 2015 | 861 | Japan | 72.1 ± 7.1 | Actiwatch 2; Respironics, Murrysville, PA, USA | Actigraphy | Actiware version 5.5 software | Sleep onset, sleep offset, SOL, SE | a. Evening indoor temperature; b. initial bed temperature | a. SOL; b. SOL |
| 31. (Tsuzuki et al., 2015) | 2015 | 8 Men | Japan | 64 ± 1 | Micro‐mini motionlogger actigraph; Ambulatory Monitoring, NY, USA and Actiwatch‐L; Mini Mitter (wrist‐worn) | Actigraphy | Action‐W, 2.4.20, Ambulatory Monitoring using the Cole–Kripke algorithm for scoring sleeping and waking | SOL, TST, SE | a. Seasonal thermal environment; b. illuminance | a. SE, WASO, TST; b. TST, WASO, SE |
| 32. (Parsey et al., 2015) | 2015 | 85 | USA | 50–86 (67.69 ± 9.90) |
Micro‐Mini Motionlogger, Ambulatory Monitoring (wrist‐worn) |
Actigraphy | Act Millennium software version 3.31 (Ambulatory Monitoring) and the Cole–Kripke default scoring algorithm | TST, SOL, SE, WASO | Everyday functioning (direct observation, self‐report and paper‐and‐pencil‐based problem‐solving tasks) | None |
| 33. (Obayashi, Saeki, & Kurumatani, 2014) | 2014 | 857 | Japan | ≥ 60, mean 72.2 |
Actiwatch 2; Respironics, (Murrysville, PA) (wrist‐worn) |
Actigraphy | Actiware version 5.5 (Respironics) with the default algorithm (Philips Respironics Actiware Tutorials, 2013) | SE, SOL, WASO, TST, sleep‐mid time, mid‐time between sleep onset and sleep offset | LAN | SE, SOL, WASO, TST, sleep mid‐time |
| 34. (Stone et al., 2014) | 2014 | 3101Men | USA | ≥ 67 (76 ± 0) | SleepWatch‐O, Ambulatory Monitoring, Ardsley, NY (wrist‐worn) | Actigraphy | Action W‐2 software, Cole–Kripke algorithm, University of California, San Diego (UCSD) scoring algorithm | TST, SE, TIB (time spent sleeping after lights off), nap time (number) | Number of falls (recurrent/non‐recurrent) | TST, SE, nap time |
| 35. (Patel et al., 2014) | 2014 | 6038 | USA | ≥ 65 | Sleepwatch‐O, Ambulatory Monitoring, Ardsley NY (wrist‐worn) | Actigraphy | Action W‐2 software (Ambulatory Monitoring) raw data, the University of California San Diego (UCSD) scoring algorithm to determine sleep/wake status | TST (variability), nap time |
BMI Obesity was defined as a BMI ≥ 30 kg m−2 in men and women |
TST variability, nap time |
| 36. (Obayashi, Saeki, Iwamoto, et al., 2014) | 2013 | 192 | Japan | ≥ 60 (69.9 ± 0) | (Actiwatch 2; Respironics, Murrysville, PA) (wrist‐worn) | Actigraphy | Actiware software (version 5.5, Respironics, Murrysville, PA) | SOL | Evening light exposure | SOL |
| 37. (Haimov & Shatil, 2013) | 2013 | 51 | USA | 65–85 |
Mini Motionlogger, Ambulatory Monitoring, Ardsley, New York, USA (wrist‐worn) |
Actigraphy | Actigraphic Scoring Analysis program for an IBM‐compatible personal computer (W2 scoring algorithm) provided by the manufacturer | TST, SOL, SE, WASO, NoA | CogniFit intervention, a cognitive training program | SOL, SE, WASO, NoA |
| 38. (Scullin, 2013) | 2013 | 41 | USA | (70.66 ± 5.41) |
Zeo (headband) |
EEG | Proprietary algorithms from the device manufacturer | Slow‐wave sleep | Episodic memory | Amount of slow‐wave sleep |
| 39. (Miyata et al., 2013) | 2013 | 78 | Japan | ≥ 60 (72.2 ± 5.9) | Ambulatory Monitoring, New York, NY, USA (wrist‐worn) | Actigraphy | Algorithm supplied by the ActionW‐2 clinical sleep analysis software package for Windows (Ambulatory Monitoring) Sleep and activity were scored according to the Cole–Kripke formula | TST, SE, SOL and WASO | a. CPT (for sustained attention and vigilance); b. working memory capacity | a. No significance; b. TST, SE |
| 40. (Spira et al., 2012) | 2013 | 817 women | USA | 82.4 ± 00.00 | SleepWatch‐O, Ambulatory Monitoring, Ardsley, NY (wrist‐worn) | Actigraphy | Action W‐2 software (Ambulatory Monitoring) | TST, WASO, SE | a. IADL impairment; b. grip strength; c. gait speed at baseline and 5‐year follow‐up | a. TST, SE; b. TST, WASO and SE; c. no significant association |
| 41. (Taibi & Vitiello, 2011) | 2011 | 13 women | USA | 55–85 (65.2 ± 6.9) |
Mini‐mitter Company, Bend, OR (wrist‐worn) |
Actigraphy | Actiware version 5.57 (Mini‐mitter Company, Bend, OR) | SOL, WASO, TST, SE | Yoga intervention | no associations |
| 42. (McCurry et al., 2011) | 2011 | 132 | USA | 82.2 ± 8.5 | Micro‐Mini Motionlogger actigraph (Ambulatory Monitoring, Ardcsley, NY) (wrist‐worn) | Actigraphy | Action4 software package (Ambulatory Monitoring), which incorporates Cole–Kripke's sleep scoring algorithm | WASO, TST, TIB, daytime sleep/inactivity | Walking, light exposure, guided sleep education (NITE‐AD) | WASO |
| 43. (Dzierzewski et al., 2010) | 2011 | 50 | USA | 60–90 (69.10 ± 7.02) | Actiwatch‐L monitors | Actigraphy | Actiware‐Sleep v. 3.3 | WASO, TST | Subjective pain | TST |
| 44. (Okamoto‐Mizuno & Tsuzuki, 2010) | 2010 | 19 | JAPAN | ≥ 62 (65.8 ± 2.6) | Actigraph Basic, Ambulatory Monitoring | Actigraph | The software Action W (AMI) | Bedtime, wakeup time, TIB, TST, SE, SOL | Outside temperature and the temperature and RH, at night skin temperature (Tsk), microclimate and bed climate were continuously measured at 1‐min intervals | TST, SE |
| 45. (Ensrud et al., 2009) | 2009 | 3133 men | USA | ≥ 65 | Ambulatory Monitoring, Ardsley, NY | Actigraphy | ActionW‐2 software (Ambulatory Monitoring, Ardsley, NY) | TST, SE, SOL, WASO, NoA | Frailty status | SE, SOL, TST, NoA, WASO |
| 46. (Friedman et al., 2009) | 2009 | 51 insomnia patients | USA | ≥ 55 (63.6 ± 7.1) | Actiwatch‐L (MiniMitter, Bend OR) | Actigraphy | Actiware‐Sleep v.3.1 software program (Mini‐ Mitter, Bend OR) | WASO, TIB, TST, SE | A 12‐week program of sleep hygiene and exposure either to a. bright (≥ 4000 lux) or b. dim light (≈65 lux) scheduled daily in the morning or evening for 45 min | a. TIB, TST; b. no sign association |
| 47. (Spira et al., 2009) | 2009 | 3040 women | USA | ≥ 65 (83.6) | (SleepWatch‐O; Ambulatory Monitoring, Ardsley, NY) (wrist‐worn) | Actigraphy | ActionW‐2 software (Ambulatory Monitoring) | TST, SE, WASO, SOL, nap time | ANX | SE, WASO |
| 48. (Barrett‐Connor et al., 2008) | 2008 | 1312 men | USA | ≥ 65 | The Octagonal Sleep Watch actigraph (Ambulatory Monitoring, Ardsley, NY) | Actigraphy | Action W software, based on an algorithm developed at the University of California, San Diego | TST, SE, SOL, WASO | Testosterone | SE, WASO |
| 49. (Martin et al., 2008) | 2008 | 38 | USA | ≥ 65 |
(Octagonal‐L; Ambulatory Monitoring [AMI], Ardsley, NY) |
Actigraphy | Action4 software; (AMI) | TIB (sleep time and wakeup time), NoA, night time percentage sleep, daytime percentage sleep | Sleep in assisted living facility, sleep at home, daytime light exposure, 1000 lux | NoA, TIB |
| 50. (Lim et al., 2013) | 2013 | 737 | CANADA | 81.6 (7.2) |
Actical (Phillips Respironics, Bend, OR) The Actical is a wristwatch‐like accelerometer |
Actigraphy | Using algorithms implemented in the MATLAB language (Mathworks, Natick, MA) | Sleep fragmentation | Incident AD and cognitive decline | Sleep fragmentation |
| 51. (Blackwell et al., 2014) | 2014 | 2822 men | USA | ≥ 65 (76.0 ± 5.3) | SleepWatch‐O, Ambulatory Monitoring, Ardsley, NY (wrist‐worn) | Actigraphy | ActionW‐2 software (Ambulatory Monitoring, Ardsley, NY) | TST, SE, WASO, NoA | Cognitive function decline | WASO, NoA, SE |
| 52. (Misti L. Paudel et al., 2009) | 2008 | 3051 men | USA | ≥ 67 | SleepWatch‐O, Ambulatory Monitoring, Ardsley, NY (wrist‐worn) | Actigraphy | ActionW‐2 software (Ambulatory Monitoring, Ardsley, NY) | SE, SOL, WASO, NoA, TST | Depressive symptoms assessed using the 15‐item GDS | SOL |
| 53. (Patel et al., 2008) | 2008 | 3055 men and 3052 women = 6107 | USA | ≥ 65 (67–99) | SleepWatch‐O, Ambulatory Monitoring, Ardsley, NY (wrist‐worn) | Actigraphy | ActionW‐2 software (Ambulatory Monitoring, Ardsley, NY) | TST | Obesity | TST |
| 54. (Maglione et al., 2008) | 2012 | 3045 women | USA | ≥ 70 | SleepWatch‐O, Ambulatory Monitoring, Ardsley, NY (wrist‐worn) | Actigraphy | ActionW‐2 software (Ambulatory Monitoring, Ardsley, NY) | TST, SOL, WASO, SE, NoA, number of naps | GDS | WASO, NoA, number of naps |
AD, Alzheimer's disease; aMCI, amnestic mild cognitive impairment; ANX, Goldberg Anxiety Scale; BCG, ballistocardiography; BMI, body mass index; CPT, continuous performance test; DTI, diffusion tensor imaging; EEG, electroencephalography; EMG, electromyography; EOG, electrooculography; EQVAS, EuroQol visual analogue scale; GDS, Geriatric Depression Scale; HC, Healthy control ; HIV, human immunodeficiency virus; IADL, instrumental activities of daily living; LAN, light at night; MCI, mild cognitive impairment; MIB, Movement in bed ; NITE‐AD, nighttime insomnia treatment and education in Alzheimer's disease; NoA, number of awakenings; NREM, non‐rapid eye movement; REM, rapid eye movement; RH, relative humidity; SD, sleep duration; SE, sleep efficiency; SL, Sleep Latency; SOL, sleep‐onset latency; SW, Slow wave ; TIB, time in bed; TST, total sleep time; WASO, wakeup after sleep onset.
Ghazi, S. N. , Behrens, A. , Berner, J. , Sanmartin Berglund, J. , & Anderberg, P. (2025). Objective sleep monitoring at home in older adults: A scoping review. Journal of Sleep Research, 34(4), e14436. 10.1111/jsr.14436
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Altendahl, M. , Cotter, D. L. , Staffaroni, A. M. , Wolf, A. , Mumford, P. , Cobigo, Y. , Casaletto, K. , Elahi, F. , Ruoff, L. , Javed, S. , Bettcher, B. M. , Fox, E. , You, M. , Saloner, R. , Neylan, T. C. , Kramer, J. H. , & Walshid, C. M. (2020). REM sleep is associated with white matter integrity in cognitively healthy, older adults. PLoS One, 15, e0235395. 10.1371/journal.pone.0235395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli‐Israel, S. (2009). Sleep and its disorders in aging populations. Sleep Medicine, 10(1), S7–S11. 10.1016/J.SLEEP.2009.07.004 [DOI] [PubMed] [Google Scholar]
- Arksey, H. , & O'Malley, L. (2007). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- Arnal, P. J. , Thorey, V. , Debellemaniere, E. , Ballard, M. E. , Bou, A. , Guillot, A. , Jourde, H. , Harris, M. , Guillard, M. , van, P. , Chennaoui, M. , & Sauvet, F. (2020). The dreem headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep, 43(11), zsaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astara, K. , Tsimpolis, A. , Kalafatakis, K. , Vavougios, G. D. , Xiromerisiou, G. , Dardiotis, E. , Christodoulou, N. G. , Samara, M. T. , & Lappas, A. S. (2023). Sleep disorders and alzheimer's disease pathophysiology: The role of the glymphatic system. A scoping review. Mechanisms of Ageing and Development, 111899. [DOI] [PubMed] [Google Scholar]
- Baron, K. G. , Duffecy, J. , Berendsen, M. A. , Cheung Mason, I. , Lattie, E. G. , & Manalo, N. C. (2018). Feeling validated yet? A scoping review of the use of consumer‐targeted wearable and mobile technology to measure and improve sleep. Sleep Medicine Reviews, 40, 151–159. 10.1016/J.SMRV.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett‐Connor, E. , Dam, T. T. , Stone, K. , Harrison, S. L. , Redline, S. , & Orwoll, E. (2008). The association of testosterone levels with overall sleep quality, sleep architecture, and sleep‐disordered breathing. Journal of Clinical Endocrinology and Metabolism, 93(7), 2602–2609. 10.1210/jc.2007-2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauducco, S. , Pillion, M. , Bartel, K. , Reynolds, C. , Kahn, M. , & Gradisar, M. (2024). A bidirectional model of sleep and technology use: A theoretical review of how much, for whom, and which mechanisms. Sleep Medicine Reviews, 76, 101933. [DOI] [PubMed] [Google Scholar]
- Behrens, A. , Anderberg, P. , & Berglund, J. S. (2023). Sleep disturbance predicts worse cognitive performance in subsequent years: A longitudinal population‐based cohort study. Archives of Gerontology and Geriatrics, 106, 104899. 10.1016/J.ARCHGER.2022.104899 [DOI] [PubMed] [Google Scholar]
- Bernstein, J. P. K. , Dorociak, K. , Mattek, N. , Leese, M. , Trapp, C. , Beattie, Z. , Kaye, J. , & Hughes, A. (2022). Unobtrusive, in‐home assessment of older adults' everyday activities and health events: Associations with cognitive performance over a brief observation period. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 29(5), 781–798. 10.1080/13825585.2021.1917503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell, T. , Yaffe, K. , Laffan, A. , Ancoli‐Israel, S. , Redline, S. , Ensrud, K. E. , Song, Y. , & Stone, K. L. (2014). Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community‐dwelling men: The MrOS sleep study. Sleep, 37(4), 655–663. 10.5665/sleep.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyneel, M. , Sanida, C. , Art, G. , Libert, W. , Cuvelier, L. , Paesmans, M. , Sergysels, R. , & Ninane, V. (2011). Sleep efficiency during sleep studies: Results of a prospective study comparing home‐based and in‐hospital polysomnography. Journal of Sleep Research, 20(1pt2), 201–206. 10.1111/j.1365-2869.2010.00859.x [DOI] [PubMed] [Google Scholar]
- Butkov, N. , & Keenan, S. A. (2017). An overview of polysomnographic technique. In Sleep disorders medicine (pp. 267–294). Springer New York. 10.1007/978-1-4939-6578-617 [DOI] [Google Scholar]
- Chellappa, S. L. , Bromundt, V. , Frey, S. , Schlote, T. , Goldblum, D. , & Cajochen, C. (2022). Cross‐sectional study of intraocular cataract lens replacement, circadian rest‐activity rhythms, and sleep quality in older adults. Sleep, 45(4), zsac027. 10.1093/sleep/zsac027 [DOI] [PubMed] [Google Scholar]
- Chen, J.‐H. , Waite, L. J. , & Lauderdale, D. S. (2015). Marriage, relationship quality, and sleep among us older adults. Journal of Health and Social Behavior, 56(3), 356–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Zhou, E. , Wang, Y. , Wu, Y. , Xu, G. , & Chen, L. (2023). The past, present, and future of sleep quality assessment and monitoring. Brain Research, 1810(January), 148333. 10.1016/j.brainres.2023.148333 [DOI] [PubMed] [Google Scholar]
- Cho, E. , Kim, S. , Hwang, S. , Kwon, E. , Heo, S. J. , Lee, J. H. , Ye, B. S. , & Kang, B. (2021). Factors associated with behavioral and psychological symptoms of dementia: Prospective observational study using actigraphy. Journal of Medical Internet Research, 23(10), 1–27. 10.2196/29001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, J. R. , Joo, E. Y. , Koo, D. L. , & Hong, S. B. (2013). Let there be no light: The effect of bedside light on sleep quality and background electroencephalographic rhythms. Sleep Medicine, 14(12), 1422–1425. [DOI] [PubMed] [Google Scholar]
- de, C. J. , Burger, P. , van, E. S. , Hermanides, J. , Nanayakkara, P. , Gemke, R. , Rutters, F. , & Stenvers, D. J. (2024). Sleep assessment using eeg‐based wearables–a systematic review. Sleep Medicine Reviews, 76, 101951. [DOI] [PubMed] [Google Scholar]
- Drogos, L. L. , Gill, S. J. , Tyndall, A. V. , Raneri, J. K. , Parboosingh, J. S. , Naef, A. , Guild, K. , Eskes, G. , Hanly, P. , & Poulin, M. (2016). Evidence of association between sleep quality and apoe ε4 in healthy older adults: A pilot study. Neurology, 87(17), 1836–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzewski, J. M. , Williams, J. M. , Roditi, D. , Marsiske, M. , McCoy, K. , McNamara, J. , Dautovich, N. , Robinson, M. , & McCrae, C. (2010). Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: Evidence of covariation over time. Journal of the American Geriatrics Society, 58(5), 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud, K. E. , Blackwell, T. L. , Redline, S. , Ancoli‐Israel, S. , Paudel, M. L. , Cawthon, P. M. , Dam, T. T. , Barrett‐Connor, E. , Leung, P. C. , Stone, K. L. , & Osteoporotic Fractures in Men Study Group . (2009). Sleep disturbances and frailty status in older community‐dwelling men. Journal of the American Geriatrics Society, 57(11), 2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiritu, J. R. D. (2008). Aging‐related sleep changes. Clinics in Geriatric Medicine, 24(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Fabbri, M. , Beracci, A. , Martoni, M. , Meneo, D. , Tonetti, L. , & Natale, V. (2021). Measuring subjective sleep quality: A review. International Journal of Environmental Research and Public Health, 18(3), 1082. 10.3390/IJERPH18031082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon, C. K. , & Karlawish, J. (2019). Is the who definition of health aging well? Frameworks for “health” after three score and ten (Vol. 109, p. 1106). American Public Health Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, L. , Zeitzer, J. M. , Kushida, C. , Zhdanova, I. , Noda, A. , Lee, T. , Schneider, B. , Guilleminault, C. , Sheikh, J. , & Yesavage, J. (2009). Scheduled bright light for treatment of insomnia in older adults. Journal of the American Geriatrics Society, 57(3), 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Full, K. M. , Gallo, L. C. , Malhotra, A. , Bellettiere, J. , Kerr, J. , Arredondo, E. , Stone, K. L. , Zaslavsky, O. , Lewis, C. E. , Lin, X. , & Lacroix, A. Z. (2020). Modeling the cardiometabolic benefits of sleep in older women: Exploring the 24‐hour day. Sleep, 43(1), 1–11. 10.1093/sleep/zsz205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Kholghi, M. , Koprinska, I. , & Zhang, Q. (2021). Association of Longitudinal sleep and next‐day indoor mobility measured via passive sensors among community‐dwelling older adults. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society (Vol. 2021, pp. 2400–2404). IEEE. 10.1109/EMBC46164.2021.9630784 [DOI] [PubMed] [Google Scholar]
- Gooneratne, N. S. , & Vitiello, M. V. (2014). Sleep in older adults: Normative changes, sleep disorders, and treatment options. Clinics in Geriatric Medicine, 30(3), 591–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov, I. , & Shatil, E. (2013). Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLoS One, 8(4), e61390. 10.1371/journal.pone.0061390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Q. , Hu, W. , Sun, N. , Chu, J. , Chen, X. , Li, T. , He, Q. , Feng, Z. , & Shen, Y. (2023). Bidirectional associations between sleep quality and grip strength and the mediating role of depression: Evidence from two nationally representative cohorts. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 78(12), 2449–2457. [DOI] [PubMed] [Google Scholar]
- Hita‐Yañez, E. , Atienza, M. , & Cantero, J. L. (2013). Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep, 36(9), 1327–1334. 10.5665/sleep.2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Bliwise, D. L. , Shah, A. , Johnson, D. A. , Clifford, G. D. , Hall, M. H. , Krafty, R. , Goldberg, J. , Sloan, R. , Ko, Y. A. , da, G. , Perez‐Alday, E. , Murrah, N. , Levantsevych, O. , Shallenberger, L. , Abdulbaki, R. , & Vaccarino, V. (2022). The temporal relationships between sleep disturbance and autonomic dysregulation: A co‐twin control study. International Journal of Cardiology, 362, 176–182. 10.1016/j.ijcard.2022.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz, S. A. (2021). A systematic review of sensing technologies for wearable sleep staging. Sensors, 21(5), 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal, S. J. , Topol, E. J. , & Steinhubl, S. R. (2019). Digitising the way to better sleep health. The Lancet, 393(10172), 639. [DOI] [PubMed] [Google Scholar]
- Ji, X. , & Liu, J. (2016). Subjective sleep measures for adolescents: A systematic review. Child: Care, Health and Development, 42(6), 825–839. 10.1111/CCH.12376 [DOI] [PubMed] [Google Scholar]
- Johnson, D. A. , Jackson, C. L. , Guo, N. , Sofer, T. , Laden, F. , & Redline, S. (2021). Perceived home sleep environment: Associations of household‐level factors and in‐bed behaviors with actigraphy‐based sleep duration and continuity in the Jackson heart sleep study. Sleep, 44(11), zsab163. 10.1093/sleep/zsab163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholghi, M. , Ellender, C. M. , Zhang, Q. , Gao, Y. , Higgins, L. , & Karunanithi, M. (2021). Home‐based sleep sensor measurements in an older Australian population: Before and during a pandemic. Sensors (Basel, Switzerland), 21(18), 5993. 10.3390/s21185993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. , Chun, C. , & Han, J. (2010). A study on bedroom environment and sleep quality in Korea, 19(1), 123–128. 10.1177/1420326X09358031 [DOI] [Google Scholar]
- Koch, S. , Haesler, E. , Tiziani, A. , & Wilson, J. (2006). Effectiveness of sleep management strategies for residents of aged care facilities: Findings of a systematic review. Journal of Clinical Nursing, 15(10), 1267–1275. [DOI] [PubMed] [Google Scholar]
- Kume, Y. , Kodama, A. , Sato, K. , Kurosawa, S. , Ishikawa, T. , & Ishikawa, S. (2016). Sleep/awake status throughout the night and circadian motor activity patterns in older nursing‐home residents with or without dementia, and older community‐dwelling people without dementia. International Psychogeriatrics, 28(12), 2001–2008. 10.1017/S1041610216000910 [DOI] [PubMed] [Google Scholar]
- Landry, G. J. , Best, J. R. , & Liu‐Ambrose, T. (2015). Measuring sleep quality in older adults: A comparison using subjective and objective methods. Frontiers in Aging Neuroscience, 7(SEP), 166. 10.3389/FNAGI.2015.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc, R. G. , Czarnecki, P. , Howard, J. , Jacelon, C. S. , & Marquard, J. (2022). Usability experience of a personal sleep monitoring device to self‐manage sleep among persons 65 years or older with self‐reported sleep disturbances. Computers, Informatics, Nursing: CIN, 40(9), 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat, B. , Loffler, K. A. , Reynolds, A. C. , Naik, G. , Vakulin, A. , Jennings, G. , Escourrou, P. , McEvoy, R. D. , Adams, R. J. , Catcheside, P. G. , & Eckert, D. J. (2023). High night‐to‐night variability in sleep apnea severity is associated with uncontrolled hypertension. NPJ Digital Medicine, 6(1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat, B. , Naik, G. , Reynolds, A. , Aishah, A. , Scott, H. , Loffler, K. A. , Vakulin, A. , Escourrou, P. , McEvoy, R. D. , Adams, R. J. , Catcheside, P. G. , & Eckert, D. J. (2022). Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 205(5), 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat, B. , Scott, H. , Manners, J. , Adams, R. , Proctor, S. , Mukherjee, S. , Catcheside, P. , Eckert, D. , Vakulin, A. , & Reynolds, A. (2023). Multi‐night measurement for diagnosis and simplified monitoring of obstructive sleep apnoea. Sleep Medicine Reviews, 72, 101843. [DOI] [PubMed] [Google Scholar]
- Leonidis, A. , Korozi, M. , Sykianaki, E. , Tsolakou, E. , Kouroumalis, V. , Ioannidi, D. , Stavridakis, A. , Antona, M. , & Stephanidis, C. (2021). Improving stress management and sleep hygiene in intelligent homes. Sensors, 21(7), 2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Vitiello, M. V. , & Gooneratne, N. S. (2022). Sleep in normal aging. Sleep Medicine Clinics, 17(2), 161–171. [DOI] [PubMed] [Google Scholar]
- Lim, A. S. , Kowgier, M. , Yu, L. , Buchman, A. S. , & Bennett, D. A. (2013). Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep, 36(7), 1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorette, A. , Hemmeter, U. , Siclari, F. , & Von Gunten, A. (2021). Sleep in the elderly. In Bassetti C., McNicholas W., Paunio T., & Peigneux P. (Eds.), Sleep medicine textbook (pp. 1003–1012). European Sleep Research Society (ESRS). [Google Scholar]
- Lustenberger, C. , Ferster, M. L. , Huwiler, S. , Brogli, L. , Werth, E. , Huber, R. , & Karlen, W. (2022). Auditory deep sleep stimulation in older adults at home: A randomized crossover trial. Communications Medicine, 2(1), 30. 10.1038/s43856-022-00096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione, J. E. , Ancoli‐Israel, S. , Peters, K. W. , Paudel, M. L. , Yaffe, K. , & Ensrud, K. E. (2008). Depressive symptoms and subjective and objective sleep in community‐dwelling older women. Journal of the American Geriatrics Society, 23(1), 1–7. 10.1111/j.1532-5415.2012.03908.x.Depressive [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan, A. , Mahajan, S. , & Tilekar, S. (2021). A pilot randomized controlled trial of interval training and sleep hygiene for improving sleep in older adults. Journal of Aging and Physical Activity, 29(6), 993–1002. 10.1123/japa.2020-0207 [DOI] [PubMed] [Google Scholar]
- Marks, A. , & Griefahn, B. (2007). Associations between noise sensitivity and sleep, subjectively evaluated sleep quality, annoyance, and performance after exposure to nocturnal traffic noise. Noise & Health, 9(34), 1–7. [DOI] [PubMed] [Google Scholar]
- Martin, J. L. , Alam, T. , Harker, J. O. , Josephson, K. R. , & Alessi, C. A. (2008). Sleep in assisted living facility residents versus home‐dwelling older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 63(12), 1407–1409. 10.1093/gerona/63.12.1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzotti, D. R. , Guindalini, C. , Moraes, W. A. , Andersen, M. L. , Cendoroglo, M. S. , Ramos, L. R. , & Tufik, S. (2014). Human longevity is associated with regular sleep patterns, maintenance of slow wave sleep, and favorable lipid profile. Frontiers in Aging Neuroscience, 6, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter, S. J. , Hagen, P. T. , Rieck, T. M. , Haider, C. R. , Holmes, D. R. , & Morgenthaler, T. I. (2022). Physiological markers of sleep quality: A scoping review. Sleep Medicine Reviews, 64, 101657. 10.1016/J.SMRV.2022.101657 [DOI] [PubMed] [Google Scholar]
- McCurry, S. M. , Pike, K. C. , Vitiello, M. V. , Logsdon, R. G. , Larson, E. B. , & Teri, L. (2011). Increasing walking and bright light exposure to improve sleep in community‐dwelling persons with alzheimer's disease: Results of a randomized, controlled trial. Journal of the American Geriatrics Society, 59(8), 1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata, S. , Noda, A. , Iwamoto, K. , Kawano, N. , Okuda, M. , & Ozaki, N. (2013). Poor sleep quality impairs cognitive performance in older adults. Journal of Sleep Research, 22(5), 535–541. 10.1111/JSR.12054 [DOI] [PubMed] [Google Scholar]
- Miyazaki, R. , Ayabe, M. , Kumahara, H. , Morimura, K. , & Inukai, Y. (2021). Effects of light‐to‐moderate intensity aerobic exercise on objectively measured sleep parameters among community‐dwelling older people. Archives of Gerontology and Geriatrics, 94, 104336. 10.1016/j.archger.2020.104336 [DOI] [PubMed] [Google Scholar]
- Mohamed, M. , Mohamed, N. , & Kim, J. G. (2023). Advancements in wearable eeg technology for improved home‐based sleep monitoring and assessment: A review. Biosensors, 13(12), 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, E. , Schumm, L. P. , McClintock, M. , Waite, L. , & Lauderdale, D. S. (2017). Sleep characteristics and daytime cortisol levels in older adults. Sleep, 40(5), zsx043. 10.1093/sleep/zsx043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayoshi, M. , Lutsey, P. L. , Benkeser, D. , Wassel, C. L. , Aaron, R. , Shahar, E. , Iso, H. , Allison, M. A. , Criqui, M. H. , & Redline, S. (2017). Artery disease: The multi‐ethic study of atherosclerosis (MESA) (pp. 467–475). Elsevier. 10.1016/j.atherosclerosis.2016.06.040.Association [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm, J. , Ghazi, A. N. , Ghazi, S. N. , & Sanmartin Berglund, J. (2024). Prediction of dementia based on older adults' sleep disturbances using machine learning. Computers in Biology and Medicine, 171, 108126. [DOI] [PubMed] [Google Scholar]
- Obayashi, K. , Saeki, K. , Iwamoto, J. , Okamoto, N. , Tomioka, K. , Nezu, S. , Ikada, Y. , & Kurumatani, N. (2014). Effect of exposure to evening light on sleep initiation in the elderly: A longitudinal analysis for repeated measurements in home settings. Chronobiology International, 31(4), 461–467. 10.3109/07420528.2013.840647 [DOI] [PubMed] [Google Scholar]
- Obayashi, K. , Saeki, K. , & Kurumatani, N. (2014). Association between light exposure at night and insomnia in the general elderly population: The HEIJO‐KYO cohort. Chronobiology International, 31(9), 976–982. 10.3109/07420528.2014.937491 [DOI] [PubMed] [Google Scholar]
- O'Donnell, D. , Silva, E. J. , MÜnch, M. , Ronda, J. M. , Wang, W. , & Duffy, J. F. (2009). Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. Journal of Sleep Research, 18(2), 254–263. 10.1111/J.1365-2869.2008.00719.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon, M. M. , Carskadon, M. A. , Guilleminault, C. , & Vitiello, M. V. (2004). Meta‐analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep, 27(7), 1255–1273. [DOI] [PubMed] [Google Scholar]
- Okamoto‐Mizuno, K. , & Tsuzuki, K. (2010). Effects of season on sleep and skin temperature in the elderly. International Journal of Biometeorology, 54(4), 401–409. 10.1007/s00484-009-0291-7 [DOI] [PubMed] [Google Scholar]
- Okoye, S. M. , Spira, A. P. , Perrin, N. A. , Schrack, J. A. , Han, H. R. , Wanigatunga, S. , Nyhuis, C. , & Szanton, S. (2021). Exterior housing conditions are associated with objective measures of poor sleep among low‐income older adults with disabilities. Sleep Health, 7(6), 731–734. 10.1016/j.sleh.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, C. , Pedersen, I. , Bergland, A. , Enders‐Slegers, M.‐J. , Jøranson, N. , Calogiuri, G. , & Ihlebæk, C. (2016). Differences in quality of life in home‐dwelling persons and nursing home residents with dementia – a cross‐sectional study. BMC Geriatrics, 16, 137. 10.1186/s12877-016-0312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, J. L. , Golkashani, H. A. , Ghorbani, S. , Wong, K. F. , Chee, N. I. , Willoughby, A. R. , & Chee, M. W. (2023). Selecting a sleep tracker from eeg‐based, iteratively improved, low‐cost multisensor, and actigraphy‐only devices. Sleep Health. [DOI] [PubMed] [Google Scholar]
- Ouzzani, M. , Hammady, H. , Fedorowicz, Z. , & Elmagarmid, A. (2016). Rayyan—A web and mobile app for systematic reviews. Systematic Reviews, 5(1), 210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pana, A. , Sourtzi, P. , Kalokairinou, A. , Pastroudis, A. , Chatzopoulos, S.‐T. , & Velonaki, V. S. (2021). Association between muscle strength and sleep quality and duration among middle‐aged and older adults: A systematic review. European Geriatric Medicine, 12, 27–44. [DOI] [PubMed] [Google Scholar]
- Parsey, C. M. , Schmitter‐Edgecombe, M. , & Belenky, G. (2015). Sleep and everyday functioning in older adulthood. Journal of Applied Gerontology, 34(1), 48–72. 10.1177/0733464812458364 [DOI] [PubMed] [Google Scholar]
- Patel, S. R. , Blackwell, T. , Redline, S. , Ancoli‐Israel, S. , Cauley, J. A. , Hillier, T. A. , Lewis, C. E. , Orwoll, E. S. , Stefanick, M. L. , Taylor, B. C. , Yaffe, K. , Stone, K. L. , Osteoporotic Fractures in Men Research Group , & Study of Osteoporotic Fractures Research Group . (2008). The association between sleep duration and obesity in older adults. International Journal of Obesity, 32(12), 1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S. R. , Hayes, A. L. , Blackwell, T. , Evans, D. S. , Ancoli‐Israel, S. , Wing, Y. K. , & Stone, K. L. (2014). The association between sleep patterns and obesity in older adults. International Journal of Obesity, 38(9), 1159–1164. 10.1038/ijo.2014.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel, M. L. , Taylor, B. C. , Diem, S. J. , Stone, K. L. , Ancoli‐Israel, S. , Redline, S. , & Ensrud, K. E. (2009). Association between depressive symptoms and sleep disturbances among community‐dwelling older men. Journal of the American Geriatrics Society, 56(7), 1228–1235. 10.1111/j.1532-5415.2008.01753.x.Association [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, D. , Peters, M. D. , Khalil, H. , McInerney, P. , Alexander, L. , Tricco, A. C. , Evans, C. , de, É. B. , Godfrey, C. M. , Pieper, D. , Saran, A. , Stern, C. , & Munn, Z. (2023). Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evidence Synthesis, 21(3), 520–532. 10.11124/JBIES-22-00123 [DOI] [PubMed] [Google Scholar]
- Ravyts, S. G. , & Dzierzewski, J. M. (2022). Sleep and healthy aging: A systematic review and path forward. Clinical Gerontologist, 47, 367–379. 10.1080/07317115.2022.2064789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawtaer, I. , Mahendran, R. , Kua, E. H. , Tan, H. P. , Tan, H. X. , Lee, T. S. , & Ng, T. P. (2020). Early detection of mild cognitive impairment with in‐home sensors to monitor behavior patterns in community‐dwelling senior citizens in Singapore: Cross‐sectional feasibility study. Journal of Medical Internet Research, 22(5), e16854. 10.2196/16854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman, K. N. , O'Brien, K. E. , Ruiz, F. S. , Rae, D. E. , Gómez‐Olivé, F. X. , von Schantz, M. , & Scheuermaier, K. (2022). Delayed circadian rhythms in older Africans living with human immunodeficiency virus (HIV). Journal of Pineal Research, 74(1), e12838. 10.1111/jpi.12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, C. , Mattek, N. , Lim, M. M. , Beattie, Z. , Dodge, H. H. , & Kaye, J. (2022). Association between mild cognitive impairment and seasonal rest‐activity patterns of older adults. Frontiers in Digital Health, 4, 809370. 10.3389/fdgth.2022.809370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocknathan, S.‐K. , Thone, W. , Xuan, Y. T. , Rong, A. Y. W. , Roy, A. K. R. , Alagusubramanian, D. , & Wai, A. A. P. (2017). Empirical evaluation of consumer eeg and actigraphy devices for home‐based sleep assessment. In 2017 international conference on orange technologies (icot) (pp. 49–52). IEEE. [Google Scholar]
- Rundo, J. V. , & Downey, R. (2019). Polysomnography. In Handbook of clinical neurology (Vol. 160, pp. 381–392). Elsevier. 10.1016/B978-0-444-64032-1.00025-4 [DOI] [PubMed] [Google Scholar]
- Sadek, I. , & Mohktari, M. (2018). Nonintrusive remote monitoring of sleep in home‐based situation. Journal of Medical Systems, 42(4), 64. [DOI] [PubMed] [Google Scholar]
- Saeki, K. , Obayashi, K. , Tone, N. , & Kurumatani, N. (2015). A warmer indoor environment in the evening and shorter sleep onset latency in winter: The HEIJO‐KYO study. Physiology and Behavior, 149, 29–34. 10.1016/j.physbeh.2015.05.022 [DOI] [PubMed] [Google Scholar]
- Schütz, N. , Saner, H. , Botros, A. , Pais, B. , Santschi, V. , Buluschek, P. , Gatica‐Perez, D. , Urwyler, P. , Müri, R. , & Nef, T. (2021). Contactless sleep monitoring for early detection of health deteriorations in community‐dwelling older adults: Exploratory study. JMIR mHealth and uHealth, 9(6), e24666. 10.2196/24666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, H. , Naik, G. , Lechat, B. , Manners, J. , Fitton, J. , Nguyen, D. P. , Hudson, A. L. , Reynolds, A. C. , Sweetman, A. , Escourrou, P. , Catcheside, P. , & Eckert, D. J. (2024). Are we getting enough sleep? Frequent irregular sleep found in an analysis of over 11 million nights of objective in‐home sleep data. Sleep Health, 10(1), 91–97. [DOI] [PubMed] [Google Scholar]
- Scullin, M. K. (2013). Sleep, memory, and aging: The link between slow‐wave sleep and episodic memory changes from younger to older adults. Psychology and Aging, 28(1), 105–114. 10.1037/a0028830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelye, A. , Mattek, N. , Howieson, D. , Riley, T. , Wild, K. , & Kaye, J. (2015). The impact of sleep on neuropsychological performance in cognitively intact older adults using a novel in‐home sensor‐based sleep assessment approach. The Clinical Neuropsychologist, 29(1), 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, R. , Gulati, S. , Kaur, A. , Sinhababu, A. , & Chakravarty, R. (2022). Research discovery and visualization using ResearchRabbit: A use case of AI in libraries. Collnet Journal of Scientometrics and Information Management, 16(2), 215–237. 10.1080/09737766.2022.2106167 [DOI] [Google Scholar]
- Smagula, S. F. , Stone, K. L. , Redline, S. , Ancoli‐Israel, S. , Barrett‐Connor, E. , Lane, N. E. , Orwoll, E. S. , Cauley, J. A. , & Osteoporotic Fractures in Men (MrOS) Research Group . (2016). Actigraphy‐and polysomnography‐measured sleep disturbances, inflammation, and mortality among older men. Psychosomatic Medicine, 78(6), 686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. , Blackwell, T. , Yaffe, K. , Ancoli‐Israel, S. , Redline, S. , & Stone, K. L. (2015). Relationships between sleep stages and changes in cognitive function in older men: The mros sleep study. Sleep, 38(3), 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira, A. P. , Covinsky, K. , Rebok, G. W. , Punjabi, N. M. , Stone, K. L. , Hillier, T. A. , Ensrud, K. , & Yaffe, K. (2012). Poor sleep quality and functional decline in older women Resmed for a multi‐center clinical trial on the effects of positive airway pressure therapy for sleep apnea in type 2 diabetics. Journal of the American Geriatrics Society, 60(6), 1092–1098. 10.1111/j.1532-5415.2012.03968.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira, A. P. , Stone, K. , Beaudreau, S. A. , Ancoli‐Israel, S. , & Yaffe, K. (2009). Anxiety symptoms and objectively measured sleep quality in older women. The American Journal of Geriatric Psychiatry, 17(2), 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr, I. , Rawson, A. , Schroeder, H. , & Joseph, N. (1939). Studies on the estimation of cardiac ouptut in man, and of abnormalities in cardiac function, from the heart's recoil and the blood's impacts;the ballistocardiogram. American Journal of Physiology‐Legacy Content, 127(1), 1–28. [Google Scholar]
- Stone, K. L. , Blackwell, T. L. , Ancoli‐Israel, S. , Cauley, J. A. , Redline, S. , Marshall, L. M. , & Ensrud, K. (2014). Sleep disturbances and risk of falls in older community‐dwelling men: The outcomes of sleep disorders in older men (mros sleep) study. Journal of the American Geriatrics Society, 62(2), 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. H. , Ma, T. , Yao, S. , Chen, Z. K. , Xu, W. D. , Jiang, X. Y. , & Wang, X. F. (2020). Associations of sleep quality and sleep duration with frailty and pre‐frailty in an elderly population Rugao longevity and ageing study. BMC Geriatrics, 20(1), 1–9. 10.1186/S12877-019-1407-5/TABLES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro, K. , Ohta, Y. , Hishikawa, N. , Nomura, E. , Wakutani, Y. , Takao, Y. , Omote, Y. , Takemoto, M. , Yamashita, T. , & Abe, K. (2020). Discrepancy of subjective and objective sleep problems in Alzheimer's disease and mild cognitive impairment detected by a home‐based sleep analysis. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia, 74, 76–80. 10.1016/j.jocn.2020.01.085 [DOI] [PubMed] [Google Scholar]
- Tai, Y. , Obayashi, K. , Yamagami, Y. , Yoshimoto, K. , Kurumatani, N. , Nishio, K. , & Saeki, K. (2021). Hot‐water bathing before bedtime and shorter sleep onset latency are accompanied by a higher distal‐proximal skin temperature gradient in older adults. Journal of Clinical Sleep Medicine, 17(6), 1257–1266. 10.5664/jcsm.9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taibi, D. M. , & Vitiello, M. V. (2011). A pilot study of gentle yoga for sleep disturbance in women with osteoarthritis. Sleep Medicine, 12(5), 512–517. 10.1016/j.sleep.2010.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto, S. , Yamamoto, H. , Yamagucki, Y. , Namba, R. , & Ouchi, Y. (2005). Obstructive sleep apnea causes systemic inflammation and metabolic syndrome. Chest, 127(3), 1074–1075. 10.1378/CHEST.127.3.1074 [DOI] [PubMed] [Google Scholar]
- Troxel, W. M. , Haas, A. , Ghosh‐Dastidar, B. , Holliday, S. B. , Richardson, A. S. , Schwartz, H. , Gary‐Webb, T. L. , Hale, L. , Buysse, D. J. , Buman, M. P. , & Dubowitz, T. (2020). Broken windows, broken zzs: Poor housing and neighborhood conditions are associated with objective measures of sleep health. Journal of Urban Health, 97(2), 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki, K. , Mori, I. , Sakoi, T. , & Kurokawa, Y. (2015). Effects of seasonal illumination and thermal environments on sleep in elderly men. Building and Environment, 88, 82–88. 10.1016/j.buildenv.2014.10.001 [DOI] [Google Scholar]
- von Ruesten, A. , Weikert, C. , Fietze, I. , & Boeing, H. (2012). Association of Sleep Duration with chronic diseases in the European prospective investigation into cancer and nutrition (EPIC)‐Potsdam study. PLoS One, 7(1), e30972. 10.1371/JOURNAL.PONE.0030972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wams, E. J. , Wilcock, G. K. , Foster, R. G. , & Wulff, K. (2017). Sleep‐wake patterns and cognition of older adults with amnestic mild cognitive impairment (aMCI): A comparison with cognitively healthy adults and moderate Alzheimer's disease patients. Current Alzheimer Research, 14(10), 1030–1041. 10.2174/1567205014666170523095634 [DOI] [PubMed] [Google Scholar]
- Warrier, A. , & Sood, A. (2021). Home‐based physiological monitoring of patients with covid‐19. Southwest Journal of Pulmonary & Critical Care, 23(3), 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, H. , Hill, E. A. , Kyle, S. D. , & Doherty, A. (2024). A systematic review of the performance of actigraphy in measuring sleep stages. Journal of Sleep Research, 33(5), e14143. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Yang, Y. , Huang, J. , Lailan, S. , & Tao, X. (2021). Correlates of objective sleep quality in older peritoneal dialysis patients. Renal Failure, 43(1), 180–187. 10.1080/0886022X.2020.1871369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Charting of data with ethnic background, gender and publishing venue.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


