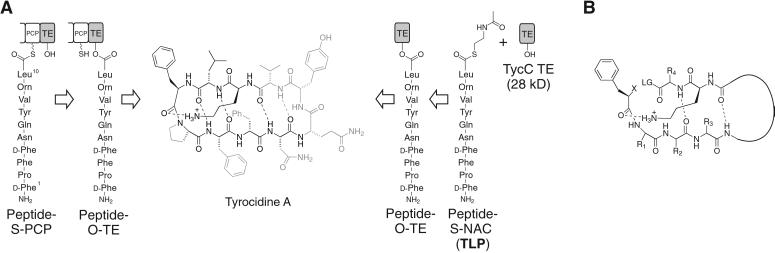
Figure 1.
(A) The natural reaction of the C-terminal TE domain from the NRPS responsible for production of the cyclic decapeptide antibiotic tyrocidine A is shown (Left). The terminal peptidyl carrier protein (PCP) is shown with its phosphopantetheine arm represented by SH. A linear decapeptide is transferred to the active-site serine (OH) of the TE domain, followed by head-to-tail cyclization giving the product tyrocidine A. (Right) The experimental design for studying the isolated TE domain. The protein-bound substrate is replaced by a decapeptide SNAC (TLP), which is cyclized by the isolated TE domain, TycC TE. The portions of the substrate that have been shown to tolerate substitution without leading to a more than 3-fold drop in the rate of cyclization are shown in gray (6, 8). (B) The proposed model for the minimal substrate, based on examination of substrates with site-specific alterations (6–8). The curved line represents a variable length linker. R represents variable side chains, X represents NH2 or OH, and LG represents a leaving group.