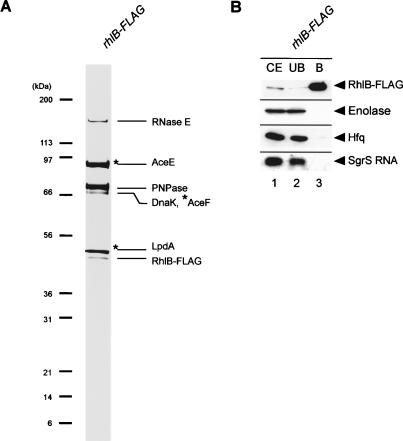
Figure 6.
(A) Protein composition of affinity-purified RhlB-Flag. TM647 (rhlB-Flag) was grown in 1 L of LB medium to A600 = 0.6. RhlB-Flag was affinity-purified and analyzed by a 4%-12% polyacrylamide gradient-0.1% SDS gel electrophoresis and CBB staining. Each of the protein bands was excised from the stained gel and identified by mass spectrometry. The positions of size markers are shown on the left.(B) Western and Northern analyses of affinity-purified Hfq-Flag. TM647 (rhlB-Flag) was grown in 200 mL of LB medium. At A600 = 0.6, 1% αMG was added and incubation was continued for 20 min. A crude extract was prepared and subjected to the pull-down assay using anti-Flag agarose. The crude extract (CE), unbound fraction (UB), and bound fraction (B) were analyzed by Western blotting. Anti-Flag, anti-enolase, and anti-HA antibodies were used to detect Hfq-Flag, enolase, and RNase E-HA (RNase E701-HA), respectively. For analysis of SgrS RNA, deproteinized crude extracts, unbound fractions, and bound fractions were subjected to Northern blotting using the sgrS probe.