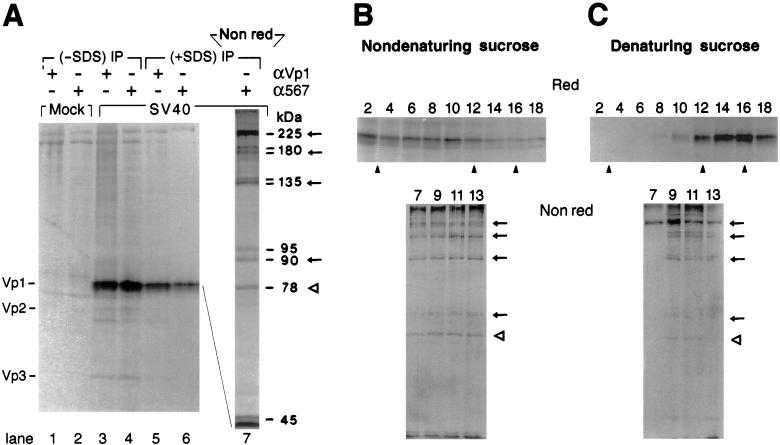
Figure 1.
Discrete, disulfide-bonded Vp1 oligomers exist in the cytoplasm. (A) SV40-infected cells at 48–50 h postinfection (lanes 3–6) or mock-infected cells (lanes 1 and 2) were labeled for 1 h and fractionated as described in Materials and Methods. The cytosolic fraction from 2.5 × 105 cells was immunoprecipitated with anti-Vp1 serum (αVp1, lanes 1, 3, and 5) or monoclonal anti-Vp1 antibody α597 (lanes 2, 4, and 6) in the absence (lanes 1–4) or presence (lanes 5–7) of SDS, eluted under reducing (lanes 1–6) or nonreducing (lane 7) conditions, and analyzed by 12.5% (lanes 1–6) or 7% (lane 7) SDS/PAGE and autoradiography. The positions for Vp1, Vp2, and Vp3 (Left) and the apparent molecular masses of labeled bands in lane 7 (Right) are indicated. Arrows mark the positions for dimeric, trimeric, tetrameric, and pentameric Vp1s, and an empty arrowhead marks the position for the 78-kDa species. (B and C) The same cytosolic fraction as in A, prepared from 2.5 × 106 cells, was sedimented through a 5–20% sucrose gradient under nondenaturing (B) or denaturing (C) conditions. Eighteen fractions were collected from the bottom of the gradient, immunoprecipitated with anti-Vp1 serum in the absence of SDS (even fractions 2–18) or with α597 in the presence of SDS (odd fractions 7–13), and analyzed by reducing (Red) or nonreducing (Nonred) 7% SDS/PAGE, respectively, followed by autoradiography. The nonreducing gels represent a three-times-longer autoradiographic exposure relative to the reducing gels. Solid arrowheads point to positions for sedimentation markers determined in parallel gradients: (from left to right) 13S, β-galactosidase; 7S, goat IgG; and 5S, ovalbumin.