Abstract
Relapse remains a major challenge for high-risk acute myeloid leukemia (AML) patients following allogeneic hematopoietic stem cell transplantation (allo-HSCT). In our first-in-human Phase I trial (ChiCTR-1900022795), we have demonstrated that third-party donor-derived double-negative T cells (DNTs) are safe and effective for treating relapsed AML. This Phase I study aims to further evaluate the safety and efficacy of allo-DNTs in preventing relapse in AML patients post-allo-HSCT. Six high-risk AML patients received three infusions of off-the-shelf allo-DNTs at one-month intervals, administered 60 to 100 days post-allo-HSCT without lymphodepleting chemotherapy. No dose-limiting toxicity, DNT-related graft-versus-host disease (GvHD), or severe cytokine release syndrome (CRS) occurred. With a median follow-up of 20.9 months (range: 11.4–24.6), four patients (66.7%) remained in minimal residual disease (MRD)-negative complete remission (CR), with recurrence-free survival exceeding 24 months. Patients in remission showed increased CD8⁺ and CD4⁺ T cells, total DNTs, and higher frequencies of granzyme-secreting T cells, which were absent in relapsed patients. In vitro, co-culturing AML patient CD8⁺ T cells with allo-DNTs upregulated granzyme B and interferon-γ expression, indicating CD8⁺ T cell activation. These findings suggest that allogeneic DNT immunotherapy is a safe, promising strategy to prevent relapse in high-risk AML patients post-allo-HSCT by combining intrinsic antitumor activity with immune modulation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-025-00680-1.
Keywords: Double negative T cell, Acute myeloid leukemia, Allogeneic hematopoietic stem cell transplantation, Relapse, Adoptive cellular therapy, Immune modulator
To the editor
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a standard treatment for high-risk acute myeloid leukemia (AML), yet about 40% of patients experience relapse [1–5]. Post-transplant interventions offer limited efficacy and carry high risks, including graft-versus-host disease (GvHD) and high treatment-related mortality [6]. Allogeneic double-negative T cells (allo-DNTs, CD3⁺CD4⁻CD8⁻), though rare (1–5% of peripheral leukocytes), show potent graft-versus-leukemia (GVL) activity without GvHD [7]. Our Phase I clinical trial (ChiCTR-1900022795) demonstrated that DNT therapy induced 50% complete remission (CR) rate in relapsed AML patients post-HSCT [8]. Here, we report a prophylactic trial of allo-DNT infusion in six AML patients post-allo-HSCT, demonstrating a favorable safety profile and early evidence supporting the potential of allogeneic DNT cell therapy as an immune-modulating approach to prevent relapse in high-risk AML patients following allo-HSCT.
Characterization of patients and DNTs
Six patients (median age 44 years; range 27–62) were enrolled (Additional file 1). The median prior treatment lines were 3 (range 1–8) before allo-HSCT (Table 1). Three patients were refractory/relapsed AML, one of which also possessed pre-transplant progressive disease and high-risk genetic mutations (IDH2 and TP53), and the other one had disease progression pre-transplantation. Two patients suffered history of myelodysplastic syndrome (MDS), one of which also had IDH2 mutation-positive pre-transplantation. The remaining one was multiparameter flow cytometry (MFC) MRD-positive pre-transplantation. One patient received haplo-HSCT, others underwent umbilical cord blood transplantation. Third-party allo-DNTs were administered without lymphodepletion, starting at a median of 92.5 days (range 64–100) post-HSCT. Each patient received three monthly infusions at escalating doses of 1 × 10⁸ and 1.5 × 10⁸ cells/kg.
Table 1.
Clinical characteristics of the patients
| Pt No. |
Dose Level (/kg) |
Age | Sex | Risk Profile | Prior treatment | Transplant type | Time between transplant and DNT infusion (days) |
Pre-infusion level (count/ml) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2022 ELN Risk Category | High risk factors for recurrence | CD4+T | CD8+ T | DNT | |||||||
| 01002 | 1 × 108 | 62 | F | Adverse |
Refractory AML; Disease progression before allo-HSCT; Genetic high-risk factors (IDH2+ & TP53+) |
Aza + Venetoclax→CR(MRD+)→Aza+ Venetoclax ×2→relapse/NR→haplo-HSCT | haplo-HSCT | 92 | 111 | 0 | 4550 |
| 01003 | 1 × 108 | 52 | M | Adverse | History of MDS |
MDS(IB-1)→secondary AML→Cladribine+ Ara-C + Venclexta→CR→UCBT |
UCBT | 100 | 245,386 | 690,142 | 26,433 |
| 01005 | 1 × 108 | 27 | F | Favorable | Refractory AML | IA→CR1→IA→Ara-C×2→relapse→M + CLAG→CR→UCBT | UCBT | 64 | 221,964 | 477,453 | 44,604 |
| 01007 | 1.5 × 108 | 35 | M | Adverse | MRD positive before allo-HSCT | IA→CR→IA→HHT + Ara-C→Ara-C×2→UCBT (CBFβ-MYH11+) | UCBT | 93 | 3195 | 3901 | 111 |
| 01008 | 1.5 × 108 | 35 | M | Adverse |
Refractory AML; Disease progression before allo-HSCT; |
IA→CR1→IA→Ara-C×2→HHT + Ara-C→relapse→IDA + CLAG→NR→IDA + CLAG→NR→UCBT | UCBT | 96 | 159,730 | 389,614 | 2285 |
| 01009 | 1.5 × 108 | 53 | F | Adverse | History of MDS; MRD positive before allo-HSCT | Venetoclax (IDH2 0.64%)→CR (IDH2+)→IA→Ara-C→MRD+ (IDH2+)→UCBT | UCBT | 85 | 192,616 | 510,483 | 6472 |
Abbreviations: IDH2, Isocitrate Dehydrogenase 2; haplo-HSCT, haploidentical hematopoietic stem cell transplantation; UCBT, umbilical cord blood transplantation; r/r AML, relapsed/refractory Acute Myeloid Leukemia; Aza, Azacitidine; Ara-C, Cytarabine; IA, Idarubicin (IDA) & Cytarabine; M + CLAG, Mitoxantrone & Cladribine & Cytarabine & G-CSF (granulocyte colony stimulating factor); CR, Complete remission; MDS-IB, Myelodysplastic syndromes with increased blasts; M, Male; F, Famale; NR, Non-remission; MRD, minimal residual disease; Pt, patient; ELN, European leukemia net; DNT, double negative T cell
Safety profiles
Mild infusion reactions (fever, headache, hypertension) occurred in 72.2% (13/18) of infusions, resolving within 24 h. Most patients experienced transient IL-6 and IL-10 elevation (Fig. 1B, Additional file 2: Figure S1). One patient developed mild cytokine release syndrome six days post-first infusion and resolved spontaneously within a week; another reported mild joint pain and resolved without intervention. A third developed toxic erythema on the lower limbs 34 days post-infusion, improving with topical glucocorticoids. Patient #01003 experienced mild chronic GvHD during the second infusion, which resolved without intervention and was thought to be unrelated to DNT infusion. No cases of DNT-related acute GvHD or neurotoxicity were observed (Additional file 2: Table S1).
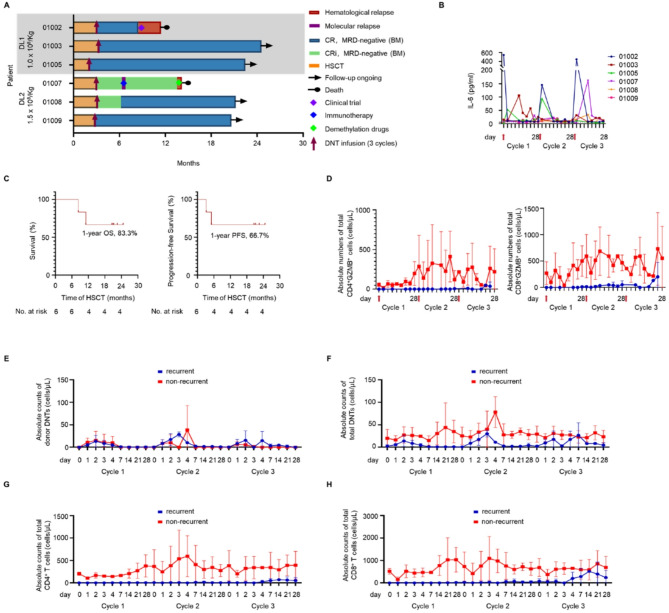
Fig. 1.
Clinical outcome and kinetics of peripheral blood biomarkers following prophylactic infusion of allo-DNTs. (A) Swimmer plots showing the clinical outcomes of six patients after three doses of allo-DNTs treatment. CRi: incomplete CR. (B) Measurement of plasma IL-6. (C) Kaplan–Meier curves showing predicted 1-year OS and 1-year PFS post HSCT. (D) Total absolute number of GZMB+ CD4+ T and CD8+ T cells. (E-H) Absolute counts of donor-DNTs (E), total DNTs (F), recipient-CD4+ T cells (G), and recipient -CD8+ T cells (H)
Clinical outcomes
As of May 1, 2025, with a median follow-up of 20.9 months (range: 11.4–24.6), four patients (66.7%) maintained MRD-negative CR without further treatment (Additional file 2: Table S2), with the longest recurrence-free survival exceeding 24 months (Fig. 1A). The 1-year OS and progression-free survival (PFS) were 83.3% and 66.7%, respectively, and the 1-year relapse incidence was 33.3% (95% CI: 9.6–80.5%) (Fig. 1C), with no non-relapse mortality. Patient #01007 experienced molecular relapse 28 days post-final infusion, achieved MRD-negativity with interferon α2b, but relapsed and died 14.2 months post-HSCT. Patient #01002, with TP53 and IDH2 mutations, complex karyotype (43–46, XX, -3, del (5) (q11q31)), del(7q10), -17), experienced pre-transplant disease progression, relapsed 8.1 months post-HSCT and died.
PK and immunomodulatory functions of allo-DNTs in patients
Donor-derived DNTs were detected in peripheral blood 1–4 days post-infusion, persisting up to 28 days and, in two non-recurrent patients, up to 360 days (Fig. 1E, Additional file 2: Table S3). Non-recurrent patients exhibited higher cytokines levels and greater expansion of total and recipient-derived DNTs, CD4⁺, and CD8⁺ T cells post-infusion (Fig. 1F-H, Additional file 2: Figure S1). They had higher effector memory T cells (Tem) proportions and granzyme expression (Fig. 1D, Additional file 2: Figure S2). However, cytokine and immune cells levels were relatively low in recurrent patients. In vitro, co-culturing patient-derived CD8⁺ T cells with allo-DNTs upregulated IFN-γ and GZMB expression, immune activation-associated cytokines and pathways (Additional file 2: Figure S3, Table S4).
In conclusion, despite our small sample size and limited follow-up, this study highlights unique advantages of allo-DNT therapy over existing post-HSCT interventions. Allo-DNT infusions, administered without lymphodepleting preconditioning, minimize treatment-related toxicity and, as an off-the-shelf product, offer a ready-to-use option for relapse prevention. Additionally, DNTs possess a dual immune-regulatory function by enhancing the indirect GVL effect through CD8⁺ T cell activation while mitigating GvHD risk. Our findings underscore the promising potential of DNT cell infusions to prevent relapse in high-risk AML patients post-transplant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients who volunteered to participate in this study, their families and caregivers, the physicians and nurses who gave clinical care, and the staff who coordinated the clinical study.
Abbreviations
- AML
Acute myeloid leukemia
- allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- UCBT
Umbilical cord blood transplantation
- DNTs
Double negative T cells
- DLT
Dose-limiting toxicity
- GvHD
Graft-versus-host disease
- CRS
Cytokine release syndrome
- CR
Complete remission
- MRD
Measurable residual disease
- OS
Overall survival
- PFS
Progression-free survival
- DLI
Donor lymphocyte infusion
- GVL
Graft-versus-leukemia
- PK
Pharmacokinetics
- Tem
Effector memory T cells
- IFN-γ
Interferon-gamma
- GZM
Granzyme
- MDS
Myelodysplastic syndrome
- IDH2
Isocitrate Dehydrogenase 2
- ELN
European leukemia net
Author contributions
Guangyu Sun was responsible for patient enrollment and treatment in the clinical trial. Xingchi Chen conducted data analysis and drafted the manuscript. Tianzhong Pan performed the detection and analysis of patient specimens. Kaidi Song analyzed and interpreted patient data related to hematologic malignancies and transplantation. Haicun Xie was responsible for cell preparation. Meijuan Tu, Wen Yao, Yaxin Cheng, Ziwei Zhou, and Dongyao Wang assisted in patient treatment. Yongsheng Han, Baolin Tang, Liming Yang, and Xiaoyu Zhu designed the experiments and revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grants # U23A20453, 82270223 and 82170209), Anhui Provincial Key Research and Development Project (grant # 2022e07020015), Anhui Health Research Project (grant # AHWJ2022a011), Anhui Provincial Department of Education Scientific Research Project (2023AH010079, 2022AH030128), Anhui Provincial Natural Science Foundation (2308085J09), International Cooperation Projects in Anhui Province (2023h11020005) and Ruichuang Biotechnology Co., Ltd., Shaoxing, China.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of the First Affiliated Hospital of the University of Science and Technology of China.
Consent for publication
All authors are consent for publication.
Competing interests
Liming Yang & Haicun Xie are employed by Ruichuang Biotechnology Co., Ltd., Shaoxing, China. Liming Yang is inventor of several DNT technology-related patents and intellectual properties. The remaining authors declare no competing interests.
Footnotes
Trial registration: This Phase I study is registered on ClinicalTrials.gov (NCT05858814).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guangyu Sun, Xingchi Chen, Tianzhong Pan and Kaidi Song contributed equally to this work.
Contributor Information
Yongsheng Han, Email: hanyongsh@163.com.
Baolin Tang, Email: zwcrystalbl@163.com.
Liming Yang, Email: lyang@wyzebiotech.com.
Xiaoyu Zhu, Email: xiaoyuz@ustc.edu.cn.
References
- 1.Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transpl. 2016;51(11):1431–8. [DOI] [PubMed] [Google Scholar]
- 2.Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transpl. 2015;21(3):454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piemontese S, Boumendil A, Labopin M, Schmid C, Ciceri F, Arcese W, et al. Leukemia relapse following unmanipulated haploidentical transplantation: a risk factor analysis on behalf of the ALWP of the EBMT. J Hematol Oncol. 2019;12(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuanelli Brambilla C, Lobaugh SM, Ruiz JD, Dahi PB, Goldberg AD, Young JW et al. Relapse after Allogeneic Stem Cell Transplantation of Acute Myelogenous Leukemia and Myelodysplastic Syndrome and the Importance of Second Cellular Therapy. Transplant Cell Ther. 2021;27(9):771.e1-.e10. [DOI] [PMC free article] [PubMed]
- 5.Sauer T, Silling G, Groth C, Rosenow F, Krug U, Görlich D, et al. Treatment strategies in patients with AML or high-risk myelodysplastic syndrome relapsed after Allo-SCT. Bone Marrow Transpl. 2015;50(4):485–92. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Chang YJ, Chen J, Han M, Hu J, Hu J, et al. Consensus on the monitoring, treatment, and prevention of leukaemia relapse after allogeneic Haematopoietic stem cell transplantation in china: 2024 update. Cancer Lett. 2024;605:217264. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Kang H, Chen B, Na Y, Khatri I, Soares F, et al. Allogeneic DNT cell therapy synergizes with T cells to promote anti-leukemic activities while suppressing GvHD. J Exp Clin Cancer Res. 2025;44(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang B, Lee JB, Cheng S, Pan T, Yao W, Wang D, et al. Allogeneic double-negative T cell therapy for relapsed acute myeloid leukemia patients post allogeneic hematopoietic stem cell transplantation: A first-in-human phase I study. Am J Hematol. 2022;97(7):E264–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.