Figure 4.
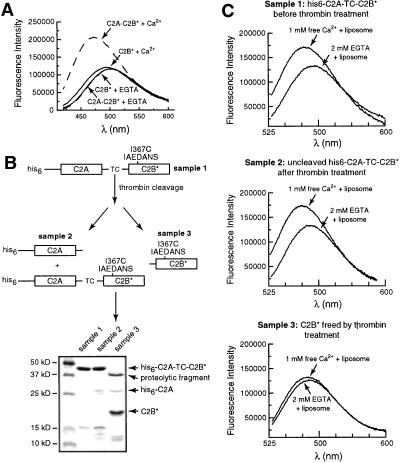
The ability of C2B to penetrate bilayers strictly depends on the presence of an adjacent C2A domain. (A) The isolated recombinant C2B domain does not penetrate into lipid bilayers. Fluorescence spectra were recorded as described in Fig. 1B; all samples contained acidic liposomes. C2A-C2B* (in 0.1 mM EGTA) served as a positive control and exhibited an increase in fluorescence upon addition of Ca2+ (0.5 mM). In contrast, isolated C2B, with the same fluorophore placed in the same position (residue 367), did not report significant changes in the presence of Ca2+ (0.5 mM)/liposomes or EGTA (0.1 mM)/liposomes. (B) Scheme showing the separation and purification of C2B* generated from C2A-C2B*. A His-6-tagged version of the cytoplasmic domain of syt, which contains a thrombin cleavage site (indicated as TC) between the C2A and C2B domains, was generated as described in Materials and Methods. This protein, his6-C2A-TC-C2B, was purified by using Ni-NTA-agarose beads. One-half of the protein immobilized on beads was eluted by using 400 mM imidazole and was designated as sample 1. Five units of thrombin was added to the other half of the bead-immobilized protein and incubated overnight at 4°C, after which the supernatant was collected and designated as sample 3. Cleavage was inefficient, and uncleaved material was eluted from the beads by first washing away the thrombin with 8 mM imidazole, followed by elution with 400 mM imidazole; this elute was designated as sample 2. Samples 1, 2, and 3 were labeled by IAEDANS as described in Materials and Methods, subjected to SDS/PAGE, and stained with Coomassie blue. The gel is shown in B (Lower). The identity of the proteins on the gel were confirmed by immunoblotting by using antibodies directed against the C2A or C2B domains of the protein (data not shown). In summary, sample 1 is his6-C2A-C2B* before thrombin treatment; sample 2 is his6-C2A-C2B* after thrombin treatment (as only 3% of the protein was cleaved, this sample is largely unchanged); and sample 3 contains C2B* that was liberated from his6-C2A-TC-C2B by thrombin treatment. (C) Removal of the C2A domain disrupts C2B–membrane interactions. The ability of the C2B* domains from samples 1, 2, and 3 (from B above) to interact with membranes was carried out as described in Fig. 1B. The fluorescence spectra obtained from samples 1, 2, and 3 are shown in Top, Middle, and Bottom, respectively (all samples contained equal molar protein, 0.5 μM). Thus, an adjacent C2A domain is essential for the Ca2+-dependent membrane penetration activity of the C2B domain.