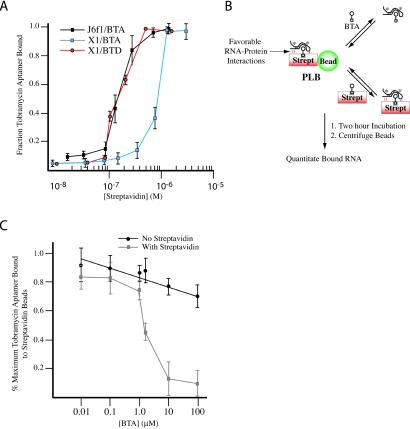
Figure 2.
Enhanced affinity of J6f1 for streptavidin mediated by BTA. (A) Binding isotherm of J6f1 and X1 to streptavidin in the presence of 2 μM BTA or 2 μM BTD. Reported values are the average of three independent experiments with SE. (B) Model of the competition-binding assay. Favorable RNA/protein-enhancing interactions are schematically denoted and predicted to result in complexes that can be more efficiently competed by streptavidin/BTA than by BTA. Asterisks denote radiolabeled RNA. (C) Competition-binding curves. Reported values represent the average of four experiments with SD shown. The maximum radioactivity associated with the beads was 11,961 cpm (100%) (PLB incubated with RNA). Less than 10% of the input radioactivity was retained if streptavidin beads were incubated with 32P-labeled J6f1 in the absence of BTA. Incubation of PLB, 32P-J6f1 RNA, and 7 μM streptavidin (no BTA) for 2 h resulted in only ≈25% of the RNA being lost from the PLB.