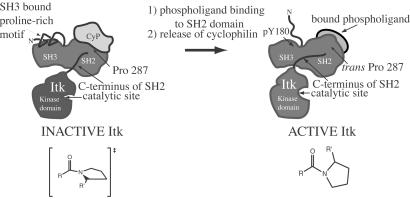
Figure 6.
Model for Itk regulation. Itk is dark gray and includes the proline-rich region, SH3, SH2, and kinase domains (the PH domain and part of the TH domain are not shown for clarity). Two “states” of Itk are depicted: inactive, CypA-bound Itk; and active, phospholigand-bound Itk. CypA is light gray and is shown bound to the Itk SH2 domain in the region of Pro-287. The proposed configuration of the Asn-286–Pro-287 imide bond (resembling the transition state for cis/trans interconversion) is illustrated for the inactive, CypA-bound form of Itk. NMR spectroscopic data suggest that cyclophilin-bound Itk is monomeric (K.N.B. and A.H.A., unpublished results), and the proline-rich region may contact the SH3 binding pocket in an intramolecular fashion (52). Activation may be accompanied by phosphorylation of Tyr-180 in the SH3 domain expelling bound proline (53). Cis/trans isomerization around Asn-286–Pro-287 causes pronounced conformational heterogeneity in the C terminus of the Itk SH2 domain leading directly into the Itk kinase domain (see Fig. 1). The C terminus of the Itk SH2 domain may adopt a conformation in the presence of cyclophilin that structurally perturbs the kinase catalytic site rendering it inactive. Release of cyclophilin, either by Itk dimerization to favor the cis conformer (not shown) or by binding of a phosphotyrosine-containing ligand to favor the trans conformer, may lead to reorganization of the C-terminal SH2 residues and subsequent restructuring of the kinase active site.