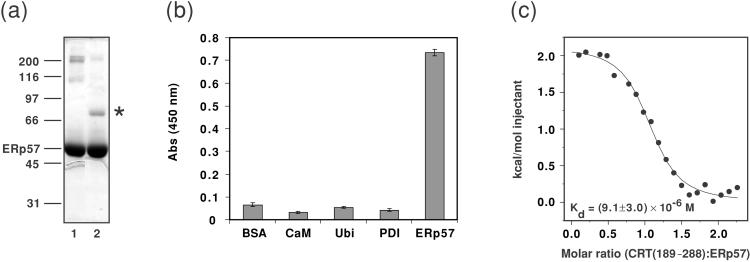
Figure 1.
(a) Chemical cross-linking of CRT(189–288) and ERp57. The homobifunctional cross-linker disuccinimidyl glutarate was added to solutions of CRT(189–288) alone (data not shown), ERp57 alone (lane 1), or an equimolar mixture of the two proteins (lane 2). After incubation for 30 min on ice, the reaction was quenched with an excess of glycine. Potential protein complexes were analyzed by 12% reducing SDS/PAGE, and the gel was stained with Coomassie blue. Molecular mass standards given in kDa are indicated on the left, along with the position of ERp57. The suggested position of the CRT(189–288)/ERp57 complex is indicated by *. (b) The interaction of CRT(189–288) and ERp57 studied by ELISA. Potential ligands for CRT(189–288) were coated in microtiter wells, and the interaction was probed with biotinylated CRT(189–288), followed by the binding of a primary antibody directed against biotin and a horseradish peroxidase-conjugated secondary antibody. Development by the addition of BM Blue (3,3′–5,5′–tetramethylbenzidine) (Roche Molecular Biochemicals) substrate gives rise to a signal at 450 nm for positive enzymatic reactions. BSA, calmodulin (CaM), and ubiquitin (Ubi) were used as control proteins. The data presented in the graph are mean values of triplicates. The experiment was repeated three times with closely similar results. Standard deviations are given by the vertical bars. Nonspecific binding of primary and secondary antibodies to wells coated with protein ligand in the absence of biotinylated CRT(189–288) was subtracted for each well. (c) Binding isotherm describing the formation of the CRT(189–288)/ERp57 complex followed by ITC at 8°C. ● represent the integrated heats at each injection after correction for nonspecific heat effects and normalization for the molar concentration. The continuous line visualizes a least-squares nonlinear fit of the data according to a 1:1 binding model defined by the following parameters: n = 1.04 ± 0.03, Kd = (9.1 ± 3.0) × 10−6 M [Ka = (1.1 ± 0.3) × 105 M−1], and ΔH = 2.1 ± 0.1 kcal mol−1.