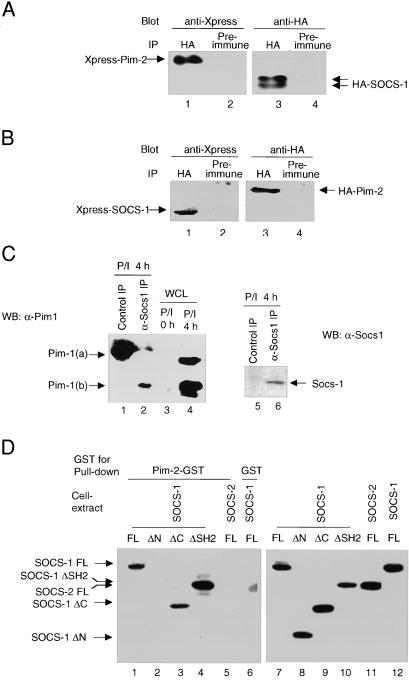
Figure 1.
Association of Pim-2 with Socs-1 in vivo and in vitro. (A) Immunoprecipitates of Socs-1 contain Pim-2. The 293T cells were cotransfected with plasmids carrying Xpress-tagged Pim-2 and HA-tagged SOCS-1. Lysates of transfectants were immunoprecipitated with a polyclonal anti-HA Ab (lanes 1 and 3) or normal rabbit serum as a negative control (lanes 2 and 4). Both immunoprecipitates were split, loaded onto SDS gel, and immunoblotted with a monoclonal anti-Xpress (lanes 1 and 2) or anti-HA (lanes 3 and 4) Abs, respectively. (B) Socs-1 is present in Pim-2 immunoprecipitates. Same as A, except that plasmids carrying Xpress-tagged SOCS-1 and HA-tagged Pim-2 were used for transfection. (C) Endogenous association of Socs-1 and Pim-1. Total thymocytes were isolated from wild-type BALB/c mice, stimulated with PMA and ionomycin for 4 h, and lysed. Lysates were immunoprecipitated by using either preimmune serum (lanes 1 and 5) or an affinity-purified rabbit α-Socs-1 antibody (lanes 2 and 6). The immunoprecipitates were analyzed by immunoblotting first with a goat α-Socs-1 antibody (C20) (Right) and then with a monoclonal α-Pim-1 antibody (Left). As a control, 50 μg of lysates from unstimulated and stimulated thymocytes was loaded in lanes 3 and 4. (D) The N terminus of Socs-1 is required for binding to Pim-2. Constructs of Xpress-tagged SOCS-1, full-length (FL) (lanes 1 and 6), ΔN (deleting amino acids 1–79) (lane 2), ΔC (deleting amino acids 167–212) (lane 3), or ΔSH2 (deleting amino acids 80–166) (lane 4) were transiently expressed in 293T cells. Xpress-tagged SOCS-2 was used as a control (lane 5). Whole-cell lysates of the transfected 293T cells were incubated with bacterial-expressed Pim-2-GST fusion protein (lanes 1–5) or GST alone (lane 6) bound to glutathione agarose beads (see Materials and Methods). Proteins bound to the beads were analyzed by immunoblot with anti-Xpress Ab. To ensure equal input, small aliquots of whole-cell lysates were subjected to Western blot analysis with anti-Xpress Ab (lanes 7–12).