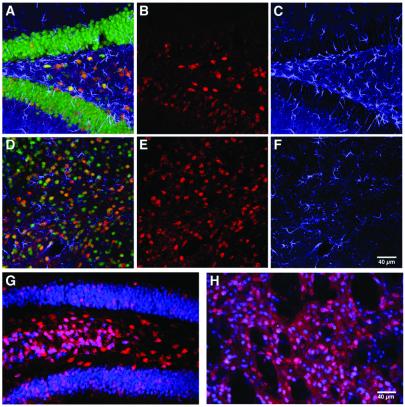
Figure 4.
Lack of significant tissue damage and inflammatory responses after AAV-GFP/Cre delivery allowing for long-term genetic modification of AAV-GFP/Cre-recombined cells. (A–F) Analysis for gliosis or recombined glial fibrillary acidic protein-positive cells 1 week postinjection of 1 × 106 infectious particles into the hippocampus (A–C) or striatum (D–F). Colocalization of β-gal (red; B and E) with glial fibrillary acidic protein staining (blue; C and F) was never detected, whereas NeuN staining (green) showed colocalization with β-gal (A and D merges). There was no evidence for gliosis occurring in either hippocampus (A and C) or striatum (D and F) in response to AAV-GFP/Cre transduction. Long-term expression of β-gal (red) was assessed at 6 months (n = 2) postinjection in the hippocampus (G) and striatum (H). Expression of β-gal (red) was colocalized with NeuN (blue). At 6 months, there was no evidence of any cell loss, vascular cuffing, or infiltrating cells indicative of toxicity.