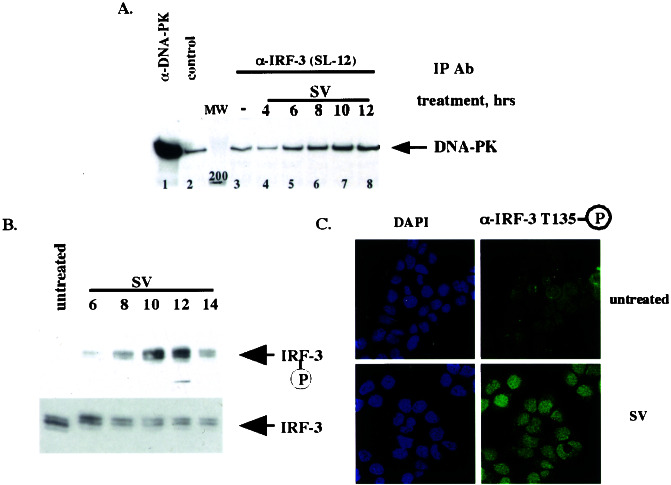
Figure 2.
DNA-PK binds and phosphorylates IRF-3 in vivo after virus infection. (A) IRF-3 and DNA-PK interact in vivo after virus infection. HEC1B cells were treated with virus (SV) for an indicated number of hours. Immunoprecipitations (IP) then were performed with control, α-DNA-PK, or α-IRF-3 (SL-12) antibody. Immunocomplexes were resolved by SDS/PAGE, and Western blotting was performed with the α-DNA-PK antibody. Fifty percent of the input is shown in the α-DNA-PK immunoprecipitation. (B) Thr-135 is phosphorylated in vivo with the kinetics similar to that of IRF-3–DNA-PK complex formation. After the treatment in A, immunoprecipitations were performed with the α-IRF-3 antibody crosslinked to protein A/G beads followed by Western blotting using affinity-purified phospho-specific antibody 2115 (Upper) or the α-IRF-3 antibody (Lower). (C) Phosphorylated IRF-3 is localized to the nucleus. HEC1B cells were treated with virus and stained with the phospho-Thr-135-specific antibody (see Materials and Methods).