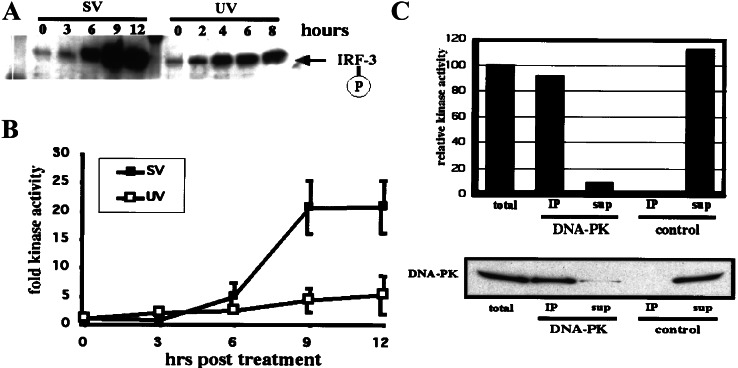
Figure 3.
Virus infection induces DNA-PK kinase activity. (A) Virus-induced phosphorylation of Thr-135 on IRF-3 occurs with faster kinetics in HT-29 cells than in HEC1B cells (compare with Fig. 2B). HT-29 cells were infected with SV for up to 12 h and harvested, and immunoprecipitations (IP) were performed as described for Fig. 2B. Phospho-Thr-135 was detected by Western blotting using affinity-purified phospho-specific antibody. Also shown is the UV-induced phosphorylation of Thr-135 on IRF-3 in HT-29 cells assayed at 2-h intervals up to 8 h. (B) HT-29 cells were infected with SV or treated with UV light and assayed at 3-h time points. Kinase activity was tested in nuclear extracts by phosphorylation of a mutant p53 peptide. Values representing three independent experiments were plotted relative to the activity in untreated extracts. (C) Virus infection activates predominantly DNA-PK. Immunoprecipitations using control antibody (normal mouse serum) or DNA-PK-specific antibody were performed with nuclear extract of HT-29 cells that had been infected with SV for 12 h. Kinase activity was determined as described above. Values were plotted relative to the control precipitation. In addition, precipitated and depleted protein was separated by 3–8% Tris-acetate PAGE. Immunoblotting was performed by using the DNA-PK-specific antibody. sup, supernatant.