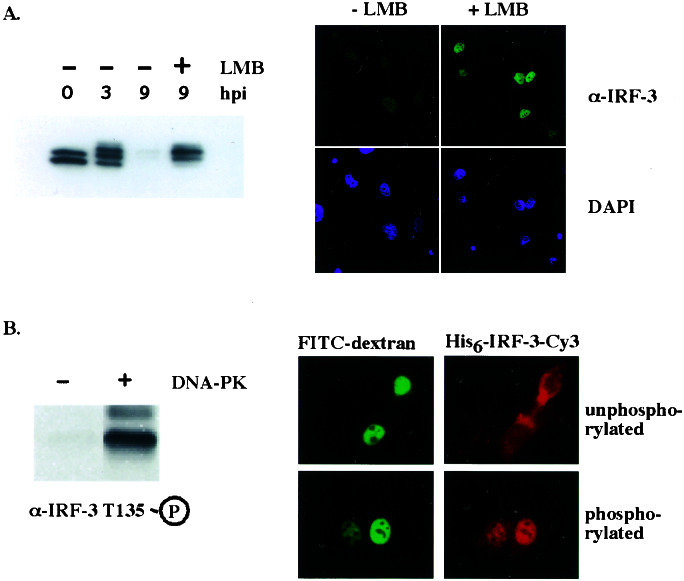
Figure 5.
Phosphorylation of Thr-135 prevents IRF-3 degradation by causing its nuclear retention. (A) Crm-1-dependent export of active IRF-3 is required for its degradation. M059J cells were infected with virus for 9 h. Three hours after the infection, when IRF-3 had been activated and accumulated in the nucleus, cells were treated with leptomycin B (LMB) or left untreated for the rest of the infection. IRF-3 protein levels (Upper) and nuclear staining (Lower) were compared as described for Fig. 4. hpi, hours postinfection. (B) Phosphorylation of IRF-3 by DNA-PK prevents its nuclear export. Bacterially produced His-tagged IRF-3 was phosphorylated with DNA-PK in vitro and fluorescently labeled with Cy3. The phosphorylated protein was microinjected into the nuclei of NIH 3T3 cells, and its export was compared with that of unphosphorylated IRF-3. FITC-conjugated dextran served as a coinjection marker.