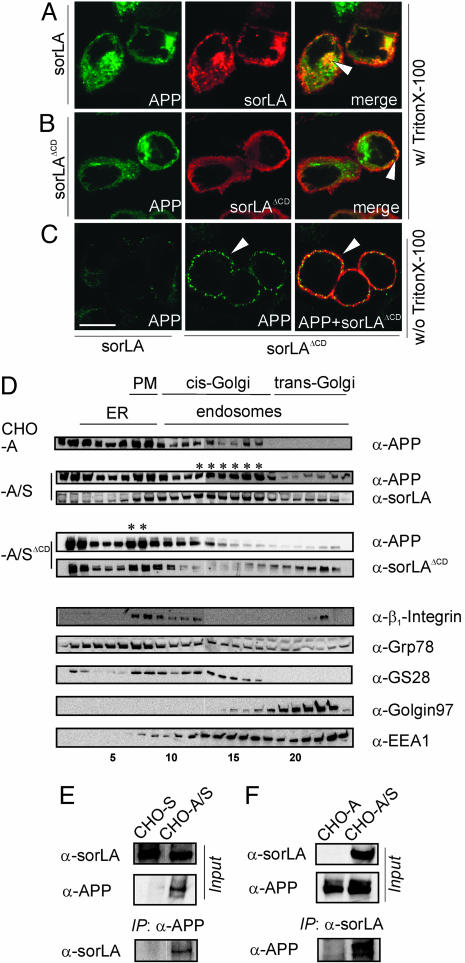
Fig. 2.
SorLA affects trafficking of APP in CHO cells. (A-C) Detection of APP695 and sorLA in CHO cells by using confocal immunofluorescence microscopy. (A) In permeabilized cells (with Triton X-100), APP695 and wild-type sorLA signals colocalize to intracellular vesicular structures and to the perinuclear region (arrowhead). (B) In stable transfectants coexpressing APP695 and sorLAΔCD, the sorLAΔCD signal is confined to the cell surface, where it colocalizes with APP (arrowhead). (C) In nonpermeabilized cells (without Triton X-100), more APP695 is detected on the surface in cells that express sorLAΔCD (Center, arrowhead), where it colocalizes with the mutant receptor (Right, arrowhead) compared with cells expressing wild-type sorLA (Left). (Scale bar: 10 μm.) (D) Subcellular fractionation of cells expressing APP695 (CHO-A) or APP695 together with wild-type sorLA (CHO-A/S) or sorLAΔCD (CHO-A/SΔCD). Immunodetection of APP and sorLA, as well as marker proteins β1-integrin (plasma membrane, PM), Grp78 (endoplasmic reticulum, ER), GS28 (cis-Golgi), Golgin97 (trans-Golgi network), and EEA1 (early endosomes) in the fractions is depicted. Asterisks mark coaccumulation of APP and sorLA in Golgi or in plasma membrane compartments of CHO-A/S and CHO-A/SΔCD cells, respectively. (E and F) Cells expressing sorLA (CHO-S), APP695 (CHO-A), or both (CHO-A/S) were treated with membrane-permeable cross-linker and proteins immunoprecipitated by using anti-APP (E) or anti-sorLA (F) antibodies. Western blots labeled “Input” show sorLA and APP in total cell extracts before immunoprecipitation. Western blots labeled “IP” demonstrate coimmunoprecipitation of sorLA in anti-APP (E) and APP in anti-sorLA (F) precipitates.