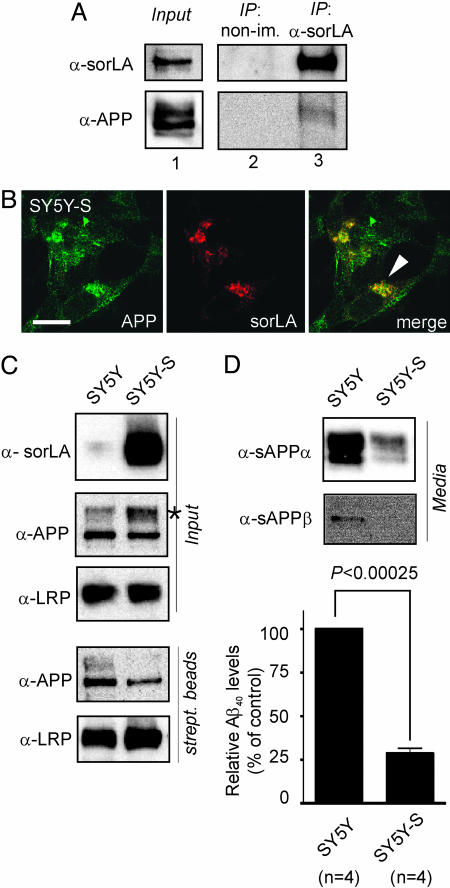
Fig. 4.
SorLA alters processing of APP in SH-SY5Y cells. (A) Nontransfected SH-SY5Y cells were treated with membrane-permeable cross-linker, and proteins were immunoprecipitated by using anti-sorLA antibodies (IP: α-sorLA) or nonimmune serum (IP: non-im.). Lane 1 shows Western blots of endogenous sorLA and APP expression in cell extracts before immunoprecipitation. Also shown are Western blots for sorLA and APP in nonimmune (lane 2) or anti-sorLA (lane 3) immunoprecipitates. (B) Immunodetection of endogenous APP and sorLA in SH-SY5Y cells transfected with a sorLA expression construct (SY5Y-S) indicating colocalization in the perinuclear region (arrowhead). (Scale bar: 10 μm.) (C) Surface biotinylation demonstrating decreased surface localization of APP in cells overexpressing sorLA. Blots labeled “Input” depict sorLA, APP, and LRP expression in cell extracts from parental (SY5Y) and sorLA-transfected (SY5Y-S) neurons. The asterisk indicates accumulation of mature APP in the presence of sorLA. Western blots labeled “strept. beads” show biotinylated APP and LRP in precipitates from streptavidin beads. Overexpression of sorLA reduces cell surface exposure of APP (35.8% of normal) but not of LRP in SY5Y-S compared with SY5Y cells. (D) Levels of sAPPα, sAPPβ, and Aβ40 in medium from SY5Y and SY5Y-S cells as determined by Western blot or ELISA. Aβ40 in SY5Y-S is reduced to 28.6 ± 3.0% (with nontransfected SY5Y levels set at 100%).