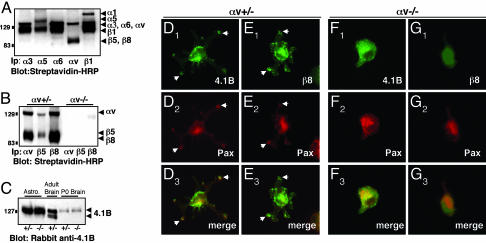
Fig. 4.
αvβ8 integrin and Band 4.1B localize to adhesion sites when astrocytes are plated on the αvβ8 ligand, LAP-TGFβ1. (A-C) Primary astrocytes express endogenous αvβ8 and Band 4.1B. (A) Astrocytes express multiple cell surface integrins as shown by immunoprecipitation with different anti-integrin antibodies. (B) Astrocytes cultured from αv+/- neonates, express αvβ5 and αvβ8, whereas αvβ5 and αvβ8 are not expressed at the cell surface of αv-/- astrocytes. (C) Protein lysates were prepared from αv+/- or αv-/- astrocytes or from neonatal brains and immunoblotted with anti-4.1B antibody. Similar levels of 4.1B protein are present in αv+/- and αv-/- samples. Adult brain lysates were immunoblotted with anti-4.1B. (D-G) Primary astrocytes cultured from neonatal mice; either αv+/- (D and E) or αv-/- (F and G) were plated on LAP-TGFβ1. Cells were immunostained with anti-4.1B or -β8 antibodies in combination with anti-paxillin to visualize adhesion sites. αv+/- astrocytes (D and E) extend multipolar processes when plated on LAP-TGFβ1. Band 4.1B (D1) and β8 integrin (E1) colocalize with paxillin (D2 and E2) in contact sites (arrows in D1-3 and E1-3). αv-/- astrocytes that do adhere to LAP-TGFβ1 do not spread and form contact sites (F1-3 and G1-3).