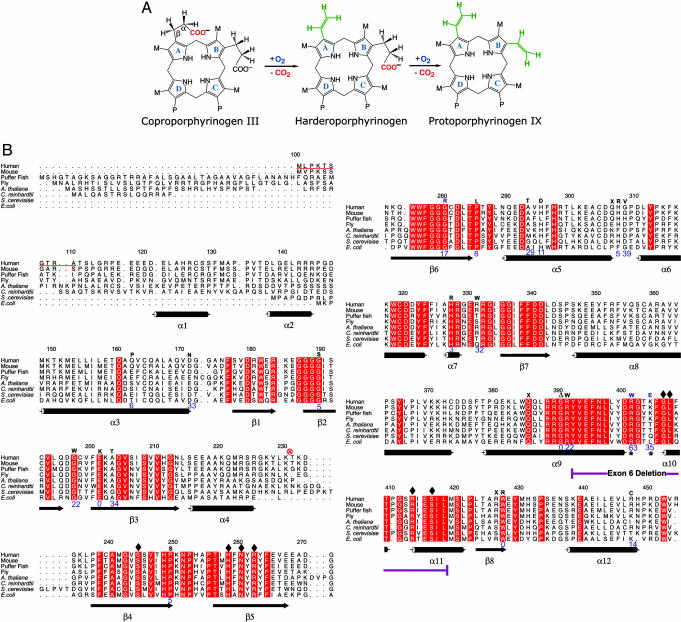
Fig. 1.
CPO chemistry and sequence conservation. (A) Reaction catalyzed by CPO involves both oxidation and decarboxylation (5). CPO sequentially decarboxylates (26, 30) the propionates attached to A and B rings without affecting those on C and D rings. A hydrogen atom from the β-position of the propionate side chain also is removed at each step (31, 32). The chemical identity of the oxidation end product(s) remains to be elucidated. M = CH3 and P = CH2.CH2.COO-.(B) Sequence alignment, secondary structure, and location of HCP-causing mutations in human CPO. The first 110 amino acids are absent in the mature enzyme, for they are part of a mitochondrial targeting signal that is cleaved upon import. In the alignment (generated by using amps and alscript) red represents absolute identity over all sequences present in that part of the alignment. Database of Secondary Structure of Proteins-derived (33) secondary structural assignments are shown directly below the alignment with cylinders indicating α-helices and arrows denoting β-strands. Mutations known to cause HCP are indicated by one letter codes above the human sequence. The enzymatic activity of these variants (24, 34) are shown in blue (% relative to native enzyme). An asterisk denotes residues that affect the second decarboxylation step. Residues that make contact with citrate are indicated by diamonds. ⊗, proteolytic cleavage site.