Fig. 1.
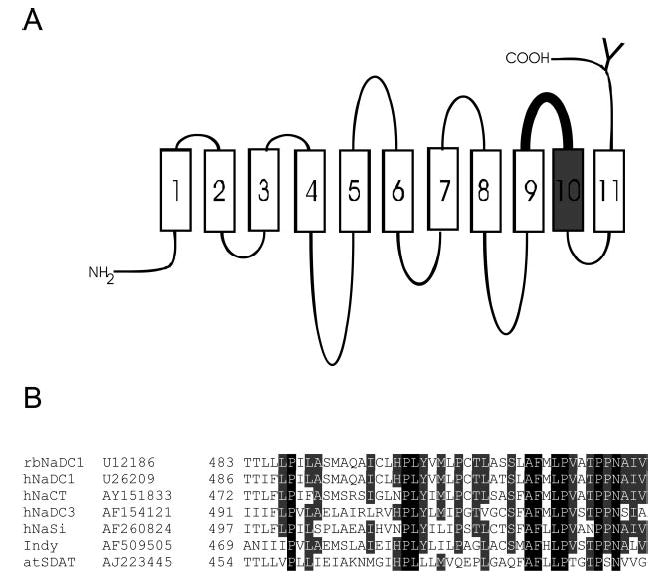
A, secondary structure model of NaDC-1 highlighting the region of the protein mutated in the cysteine scan. NaDC-1 contains 11 TMs, an intracellular amino terminus, and an extracellular carboxyl terminus. The conserved N-glycosylation site is shown by a Y. The 43 cysteine-substituted mutants in this study were made from Thr-483 to Val-528 including extracellular loop 5 (shown in bold) and TM10 (shown in gray). The outside of the cell is at the top of the figure. B, sequence alignments of the 43 amino acids mutated in this study among members of the SLC13 gene family. Names of the transporters and GenBank™ accession numbers are shown at the left. The NaDC and NaCT proteins are Na+/di- or tricarboxylate transporters, NaSi-1 is a Na+/sulfate transporter, Indy is the sodium-independent dicarboxylate exchanger from Drosophila, and atSDAT is a malate exchanger from Arabidopsis vacuoles (see reviews, Refs. 1 and 5).
