Fig. 4.
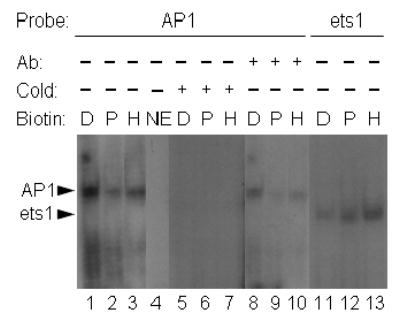
Nuclear translocation of AP1-binding proteins increases in response to biotin deficiency. HepG2 cells were cultured in media containing deficient (“D”, 0.025 nmol/L), physiological (“P”, 0.25 nmol/L), and pharmacological (“H” = high, 10 nmol/L) concentrations of biotin for 10 d. Transcription factors in nuclear extracts were probed by EMSA, using the following oligonucleotide probes and conditions: lanes 1–3 = oligonucleotide containing an AP1 consensus site; lane 4 = AP1 oligonucleotide in the absence of nuclear extract; lanes 5–7 = 50-fold molar excess of unlabeled compared to radiolabeled AP1 oligonucleotide; lanes 8–10 = AP1 oligonucleotide, supershifted by using an antibody to c-Jun; lanes 11–13 = oligonucleotide containing an ets1 site. Abbreviations: NE = no extract; Ab = antibody (supershift experiments); cold = excess of cold (unlabeled) probe.
