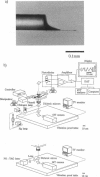
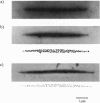
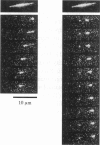
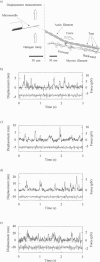
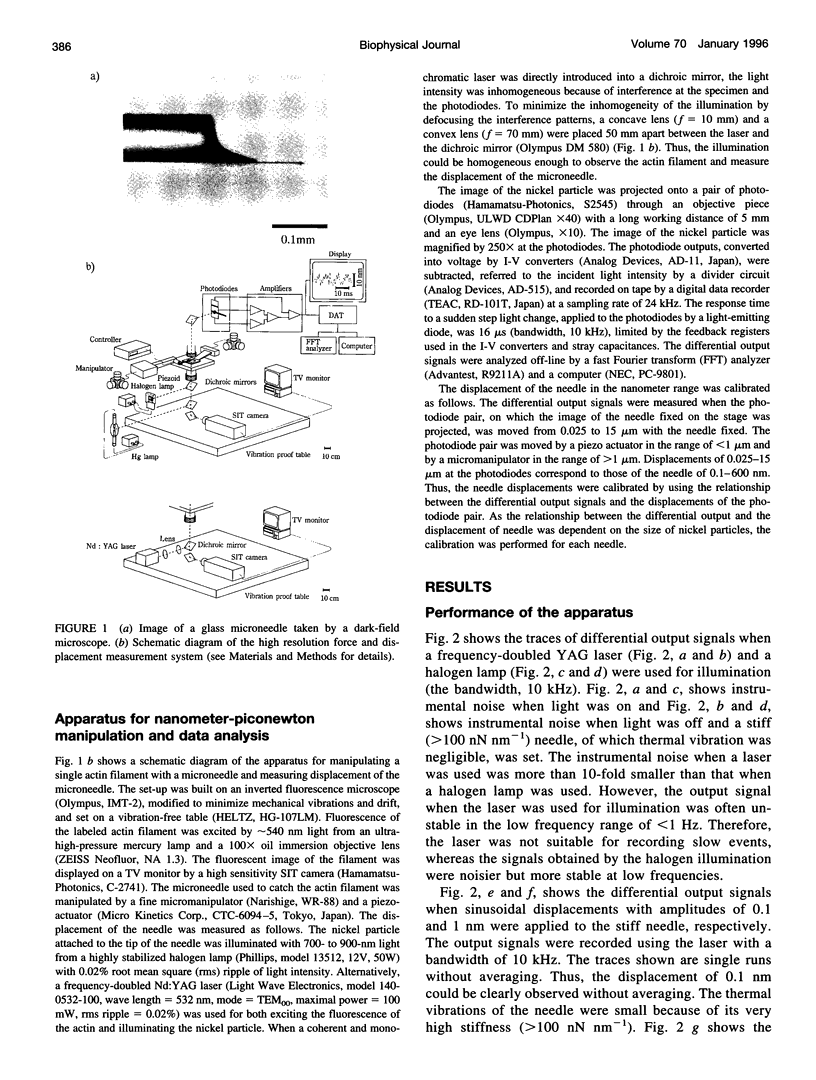
Abstract
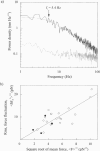
We have developed a new technique for measurements of piconewton forces and nanometer displacements in the millisecond time range caused by actin-myosin interaction in vitro by manipulating single actin filaments with a glass microneedle. Here, we describe in full the details of this method. Using this method, the elementary events in energy transduction by the actomyosin motor, driven by ATP hydrolysis, were directly recorded from multiple and single molecules. We found that not only the velocity but also the force greatly depended on the orientations of myosin relative to the actin filament axis. Therefore, to avoid the effects of random orientation of myosin and association of myosin with an artificial substrate in the surface motility assay, we measured forces and displacements by myosin molecules correctly oriented in single synthetic myosin rod cofilaments. At a high myosin-to-rod ratio, large force fluctuations were observed when the actin filament interacted in the correct orientation with a cofilament. The noise analysis of the force fluctuations caused by a small number of heads showed that the myosin head generated a force of 5.9 +/- 0.8 pN at peak and 2.1 +/- 0.4 pN on average over the whole ATPase cycle. The rate constants for transitions into (k+) and out of (k-) the force generation state and the duty ratio were 12 +/- 2 s-1, and 22 +/- 4 s-1, and 0.36 +/- 0.07, respectively. The stiffness was 0.14 pN nm-1 head-1 for slow length change (100 Hz), which would be approximately 0.28 pN nm-1 head-1 for rapid length change or in rigor. At a very low myosin-to-rod ratio, distinct actomyosin attachment, force generation (the power stroke), and detachment events were directly detected. At high load, one power stroke generated a force spike with a peak value of 5-6 pN and a duration of 50 ms (k(-)-1), which were compatible with those of individual myosin heads deduced from the force fluctuations. As the load was reduced, the force of the power stroke decreased and the needle displacement increased. At near zero load, the mean size of single displacement spikes, i.e., the unitary steps caused by correctly oriented myosin, which were corrected for the stiffness of the needle-to-myosin linkage and the randomizing effect by the thermal vibration of the needle, was approximately 20 nm.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton K. Myosin step size: estimates from motility assays and shortening muscle. J Muscle Res Cell Motil. 1992 Dec;13(6):590–607. doi: 10.1007/BF01738249. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer J. T., Mehta A. D., Spudich J. A. Characterization of single actin-myosin interactions. Biophys J. 1995 Apr;68(4 Suppl):291S–297S. [PMC free article] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu T., Harada Y., Tokunaga M., Saito K., Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995 Apr 6;374(6522):555–559. doi: 10.1038/374555a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Harada Y., Noguchi A., Kishino A., Yanagida T. Sliding movement of single actin filaments on one-headed myosin filaments. Nature. 1987 Apr 23;326(6115):805–808. doi: 10.1038/326805a0. [DOI] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990 Nov 5;216(1):49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Higashi-Fujime S. Reconstitution of active movement in vitro based on the actin-myosin interaction. Int Rev Cytol. 1991;125:95–138. doi: 10.1016/s0074-7696(08)61217-6. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Goldman Y. E. Sliding distance between actin and myosin filaments per ATP molecule hydrolysed in skinned muscle fibres. Nature. 1991 Jul 25;352(6333):352–354. doi: 10.1038/352352a0. [DOI] [PubMed] [Google Scholar]
- Horiuti K., Sakoda T., Yamada K. Mechanical response to photolytic ATP pulses of skinned muscle fibres pre-activated with a small pulse of ATP. J Muscle Res Cell Motil. 1993 Jun;14(3):335–340. doi: 10.1007/BF00123098. [DOI] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J., Vale R. D. Movement of microtubules by single kinesin molecules. Nature. 1989 Nov 9;342(6246):154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Sliding filaments and molecular motile systems. J Biol Chem. 1990 May 25;265(15):8347–8350. [PubMed] [Google Scholar]
- Ishijima A., Doi T., Sakurada K., Yanagida T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature. 1991 Jul 25;352(6333):301–306. doi: 10.1038/352301a0. [DOI] [PubMed] [Google Scholar]
- Ishijima A., Harada Y., Kojima H., Funatsu T., Higuchi H., Yanagida T. Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem Biophys Res Commun. 1994 Mar 15;199(2):1057–1063. doi: 10.1006/bbrc.1994.1336. [DOI] [PubMed] [Google Scholar]
- Itakura S., Yamakawa H., Toyoshima Y. Y., Ishijima A., Kojima T., Harada Y., Yanagida T., Wakabayashi T., Sutoh K. Force-generating domain of myosin motor. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1504–1510. doi: 10.1006/bbrc.1993.2422. [DOI] [PubMed] [Google Scholar]
- Johara M., Toyoshima Y. Y., Ishijima A., Kojima H., Yanagida T., Sutoh K. Charge-reversion mutagenesis of Dictyostelium actin to map the surface recognized by myosin during ATP-driven sliding motion. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2127–2131. doi: 10.1073/pnas.90.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Membrane noise produced by acetylcholine. Nature. 1970 Jun 6;226(5249):962–963. doi: 10.1038/226962a0. [DOI] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980 Sep;1(3):279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature. 1993 Sep 30;365(6445):454–456. doi: 10.1038/365454a0. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Yagi N., Hashizume H. Use of an X-ray television for diffraction of the frog striated muscle. Nature. 1975 Jun 26;255(5511):728–729. doi: 10.1038/255728a0. [DOI] [PubMed] [Google Scholar]
- Miyata H., Hakozaki H., Yoshikawa H., Suzuki N., Kinosita K., Jr, Nishizaka T., Ishiwata S. Stepwise motion of an actin filament over a small number of heavy meromyosin molecules is revealed in an in vitro motility assay. J Biochem. 1994 Apr;115(4):644–647. doi: 10.1093/oxfordjournals.jbchem.a124389. [DOI] [PubMed] [Google Scholar]
- Miyata H., Yoshikawa H., Hakozaki H., Suzuki N., Furuno T., Ikegami A., Kinosita K., Jr, Nishizaka T., Ishiwata S. Mechanical measurements of single actomyosin motor force. Biophys J. 1995 Apr;68(4 Suppl):286S–290S. [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Nishizaka T., Yagi T., Tanaka Y., Ishiwata S. Right-handed rotation of an actin filament in an in vitro motile system. Nature. 1993 Jan 21;361(6409):269–271. doi: 10.1038/361269a0. [DOI] [PubMed] [Google Scholar]
- Nonomura Y. Fine structure of the thick filament in molluscan catch muscle. J Mol Biol. 1974 Sep 15;88(2):445–455. doi: 10.1016/0022-2836(74)90494-x. [DOI] [PubMed] [Google Scholar]
- Prochniewicz E., Yanagida T. Inhibition of sliding movement of F-actin by crosslinking emphasizes the role of actin structure in the mechanism of motility. J Mol Biol. 1990 Dec 5;216(3):761–772. doi: 10.1016/0022-2836(90)90397-5. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Saito K., Aoki T., Aoki T., Yanagida T. Movement of single myosin filaments and myosin step size on an actin filament suspended in solution by a laser trap. Biophys J. 1994 Mar;66(3 Pt 1):769–777. doi: 10.1016/s0006-3495(94)80853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J. R., Kachar B. Polarity and velocity of sliding filaments: control of direction by actin and of speed by myosin. Science. 1990 Jul 27;249(4967):406–408. doi: 10.1126/science.2377894. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. Inferences about membrane properties from electrical noise measurements. Biophys J. 1972 Aug;12(8):1028–1047. doi: 10.1016/S0006-3495(72)86141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K., Ando M., Sutoh K., Toyoshima Y. Y. Site-directed mutations of Dictyostelium actin: disruption of a negative charge cluster at the N terminus. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7711–7714. doi: 10.1073/pnas.88.17.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. Force and velocity measured for single kinesin molecules. Cell. 1994 Jun 3;77(5):773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Tawada K., Kimura M. Stiffness of carbodiimide-crosslinked glycerinated muscle fibres in rigor and relaxing solutions at high salt concentrations. J Muscle Res Cell Motil. 1986 Aug;7(4):339–350. doi: 10.1007/BF01753655. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., Spudich J. A. The myosin step size: measurement of the unit displacement per ATP hydrolyzed in an in vitro assay. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7130–7134. doi: 10.1073/pnas.87.18.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregear R. T., Squire J. M. Myosin content and filament structure in smooth and striated muscle. J Mol Biol. 1973 Jun 25;77(2):279–290. doi: 10.1016/0022-2836(73)90336-7. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Kron S. J., Spudich J. A. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. J Mol Biol. 1990 Aug 5;214(3):699–710. doi: 10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Warrick H. M., Kron S. J., Spudich J. A. Quantized velocities at low myosin densities in an in vitro motility assay. Nature. 1991 Jul 25;352(6333):307–311. doi: 10.1038/352307a0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Tokunaga M., Kohno I., Sugimoto Y., Hamanaka T., Takezawa Y., Wakabayashi T., Amemiya Y. Small-angle synchrotron x-ray scattering reveals distinct shape changes of the myosin head during hydrolysis of ATP. Science. 1992 Oct 16;258(5081):443–447. doi: 10.1126/science.1411537. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Yamada A., Ishii N., Takahashi K. Direction and speed of actin filaments moving along thick filaments isolated from molluscan smooth muscle. J Biochem. 1990 Sep;108(3):341–343. doi: 10.1093/oxfordjournals.jbchem.a123203. [DOI] [PubMed] [Google Scholar]
- Yamada A., Wakabayashi T. Movement of actin away from the center of reconstituted rabbit myosin filament is slower than in the opposite direction. Biophys J. 1993 Feb;64(2):565–569. doi: 10.1016/S0006-3495(93)81388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida T., Harada Y., Ishijima A. Nano-manipulation of actomyosin molecular motors in vitro: a new working principle. Trends Biochem Sci. 1993 Sep;18(9):319–324. doi: 10.1016/0968-0004(93)90064-t. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Ishijima A. Forces and steps generated by single myosin molecules. Biophys J. 1995 Apr;68(4 Suppl):312S–320S. [PMC free article] [PubMed] [Google Scholar]
- Yanagida T., Nakase M., Nishiyama K., Oosawa F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature. 1984 Jan 5;307(5946):58–60. doi: 10.1038/307058a0. [DOI] [PubMed] [Google Scholar]