Abstract
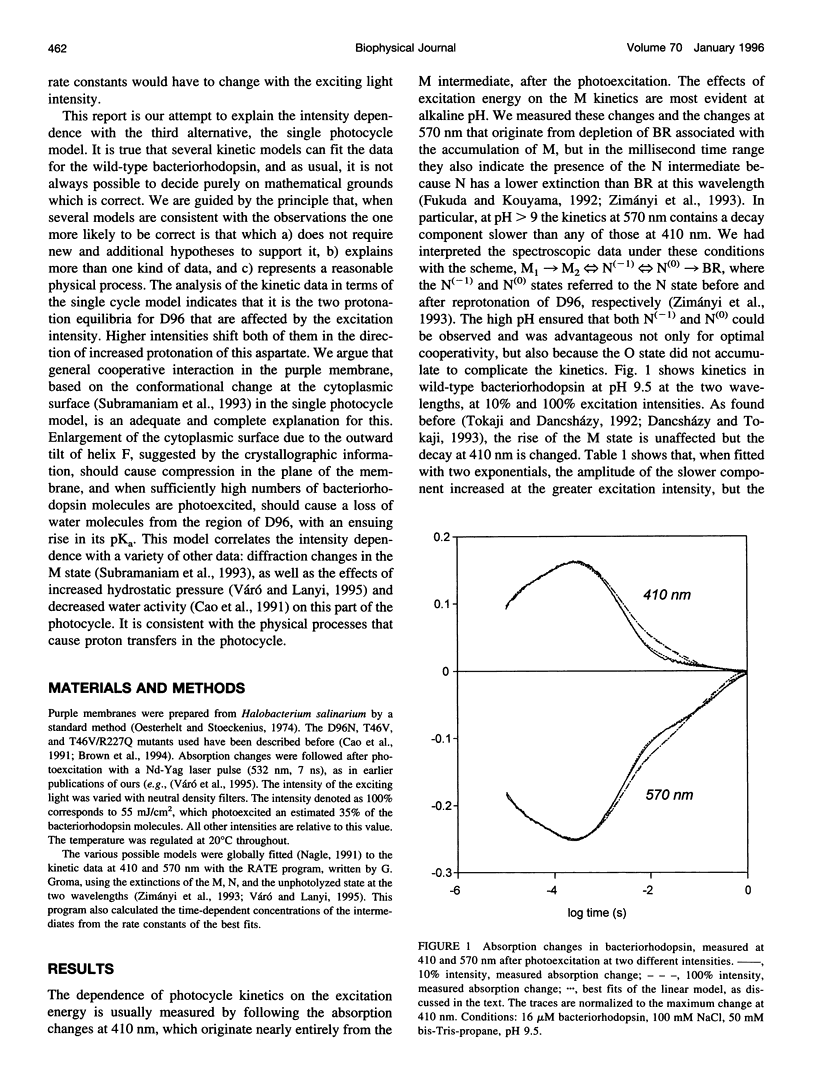
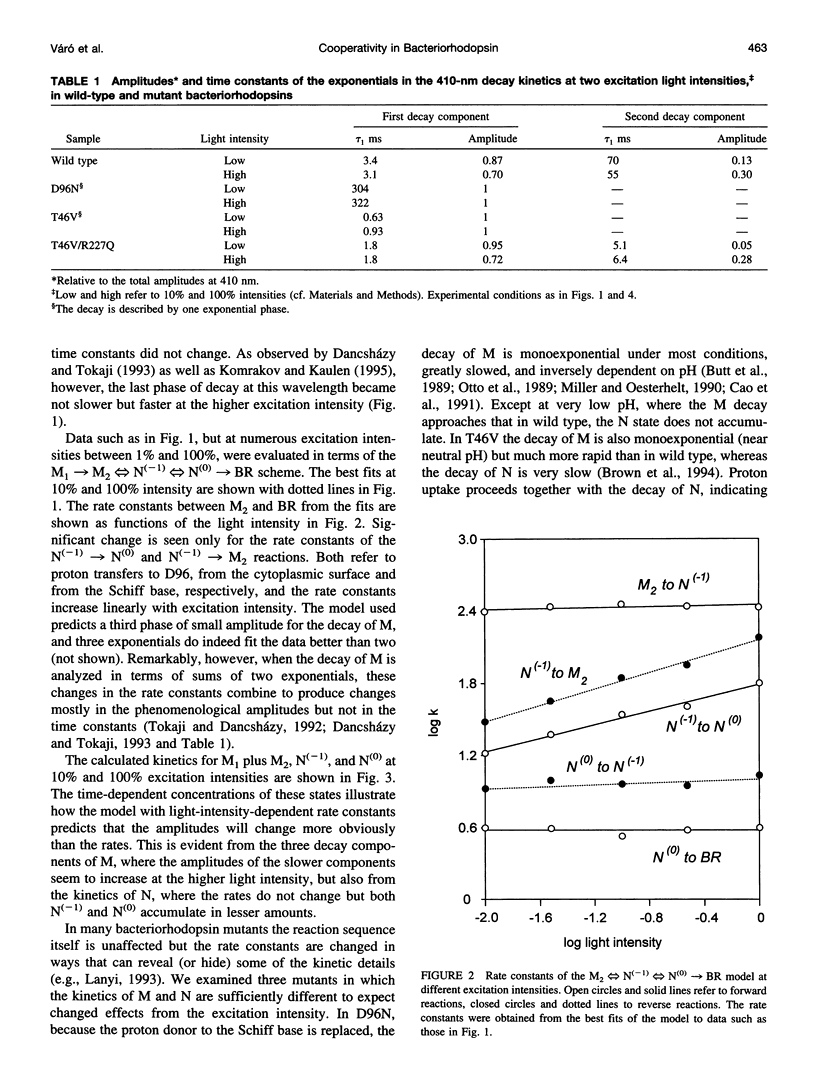
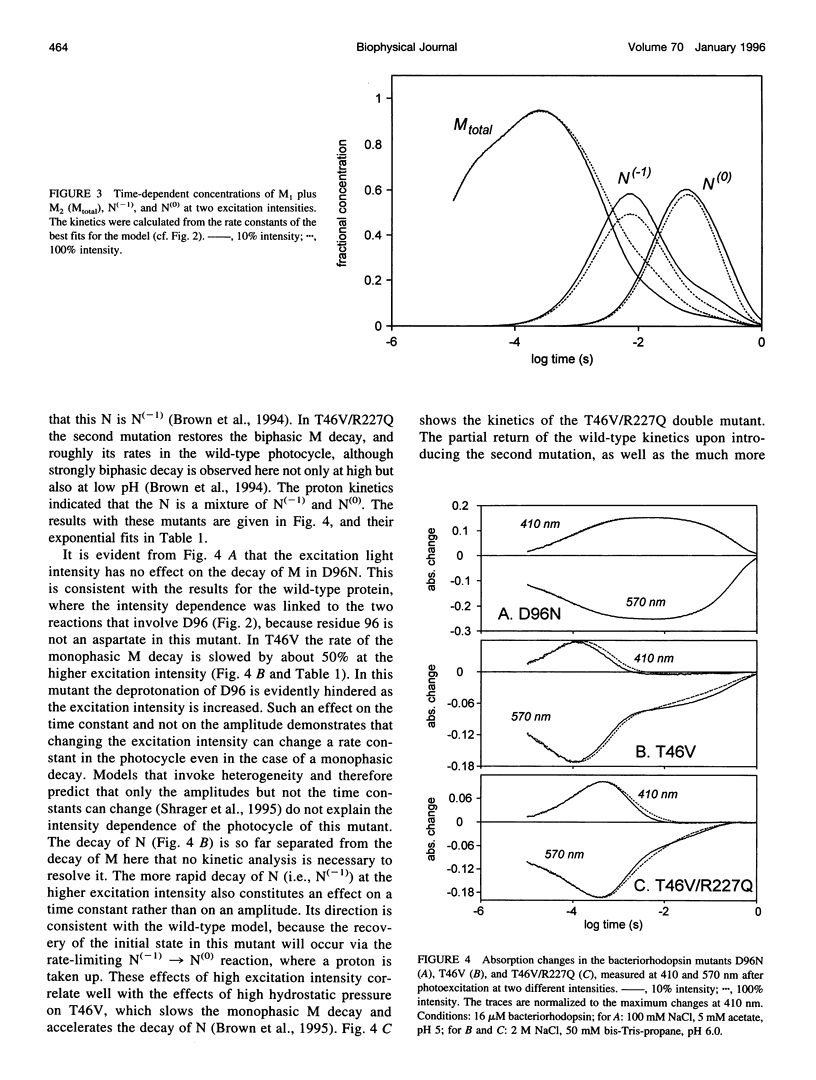
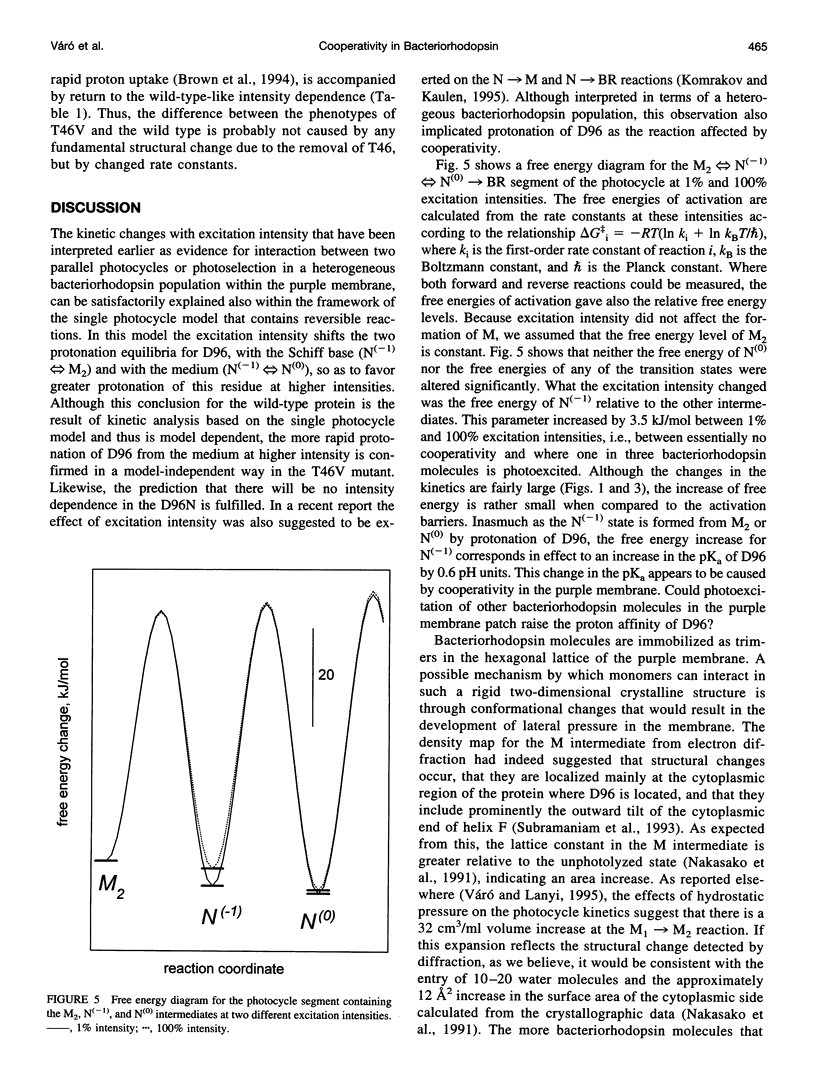
The effects of excitation light intensity on the kinetics of the bacteriorhodopsin photocycle were investigated. The earlier reported intensity-dependent changes at 410 and 570 nm are explained by parallel increases in two of the rate constants, for proton transfers to D96 from the Schiff base and from the cytoplasmic surface, without changes in the others, as the photoexcited fraction is increased. Thus, it appears that the pKa of D96 is raised by a cooperative effect within the purple membrane. This interpretation of the wild-type kinetics was confirmed by results with several mutant proteins, where the rates are well separated in time and a model-dependent analysis is unnecessary. Based on earlier results that demonstrated a structural change of the protein after deprotonation of the Schiff base that increases the area of the cytoplasmic surface, and the effects of high hydrostatic pressure and lowered water activity on the photocycle steps in question, we suggest that the pKa of D96 is raised by a lateral pressure that develops when other bacteriorhodopsin molecules are photoexcited within the two-dimensional lattice of the purple membrane. Expulsion of no more than a few water molecules bound near D96 by this pressure would account for the calculated increase of 0.6 units in the pKa.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E. Evidence of bilayer structure and of membrane interactions from X-ray diffraction analysis. Biochim Biophys Acta. 1982 May 12;650(4):167–207. doi: 10.1016/0304-4157(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Brown L. S., Váró G., Needleman R., Lanyi J. K. Functional significance of a protein conformation change at the cytoplasmic end of helix F during the bacteriorhodopsin photocycle. Biophys J. 1995 Nov;69(5):2103–2111. doi: 10.1016/S0006-3495(95)80081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. S., Yamazaki Y., Maeda A., Sun L., Needleman R., Lanyi J. K. The proton transfers in the cytoplasmic domain of bacteriorhodopsin are facilitated by a cluster of interacting residues. J Mol Biol. 1994 Jun 10;239(3):401–414. doi: 10.1006/jmbi.1994.1381. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Váró G., Chang M., Ni B. F., Needleman R., Lanyi J. K. Water is required for proton transfer from aspartate-96 to the bacteriorhodopsin Schiff base. Biochemistry. 1991 Nov 12;30(45):10972–10979. doi: 10.1021/bi00109a023. [DOI] [PubMed] [Google Scholar]
- Czégé J., Reinisch L. Cross-correlated photon scattering during the photocycle of bacteriorhodopsin. Biophys J. 1990 Sep;58(3):721–729. doi: 10.1016/S0006-3495(90)82415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancsházy Z., Tokaji Z. Actinic light density dependence of the bacteriorhodopsin protocycle. Biophys J. 1993 Aug;65(2):823–831. doi: 10.1016/S0006-3495(93)81115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Heyn M. P. Bacteriorhodopsin monomers pump protons. FEBS Lett. 1979 Dec 15;108(2):307–310. doi: 10.1016/0014-5793(79)80552-9. [DOI] [PubMed] [Google Scholar]
- Fisher K. A., Stoeckenius W. Freeze-fractured purple membrane particles: protein content. Science. 1977 Jul 1;197(4298):72–74. doi: 10.1126/science.867052. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kouyama T. Photoreaction of bacteriorhodopsin at high pH: origins of the slow decay component of M. Biochemistry. 1992 Dec 1;31(47):11740–11747. doi: 10.1021/bi00162a010. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Altenbach C., Hubbell W. L., Khorana H. G. Locations of Arg-82, Asp-85, and Asp-96 in helix C of bacteriorhodopsin relative to the aqueous boundaries. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8626–8630. doi: 10.1073/pnas.88.19.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Dancsházy Z., Bose S., Shrager R. I., Tokaji Z. Influence of excitation energy on the bacteriorhodopsin photocycle. Biochemistry. 1994 Apr 19;33(15):4604–4610. doi: 10.1021/bi00181a022. [DOI] [PubMed] [Google Scholar]
- Heremans K. High pressure effects on proteins and other biomolecules. Annu Rev Biophys Bioeng. 1982;11:1–21. doi: 10.1146/annurev.bb.11.060182.000245. [DOI] [PubMed] [Google Scholar]
- Komrakov A. Y., Kaulen A. D. M-decay in the bacteriorhodopsin photocycle: effect of cooperativity and pH. Biophys Chem. 1995 Sep-Oct;56(1-2):113–119. doi: 10.1016/0301-4622(95)00022-p. [DOI] [PubMed] [Google Scholar]
- Krebs M. P., Khorana H. G. Mechanism of light-dependent proton translocation by bacteriorhodopsin. J Bacteriol. 1993 Mar;175(6):1555–1560. doi: 10.1128/jb.175.6.1555-1560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz I. D. Hydration of macromolecules. IV. Polypeptide conformation in frozen solutions. J Am Chem Soc. 1971 Jan 27;93(2):516–518. doi: 10.1021/ja00731a037. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A. K., Bose S., Hendler R. W. Membrane-mediated control of the bacteriorhodopsin photocycle. Biochemistry. 1994 Sep 13;33(36):10889–10895. doi: 10.1021/bi00202a007. [DOI] [PubMed] [Google Scholar]
- Nagle J. F. Solving complex photocycle kinetics. Theory and direct method. Biophys J. 1991 Feb;59(2):476–487. doi: 10.1016/S0006-3495(91)82241-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasako M., Kataoka M., Amemiya Y., Tokunaga F. Crystallographic characterization by X-ray diffraction of the M-intermediate from the photo-cycle of bacteriorhodopsin at room temperature. FEBS Lett. 1991 Nov 4;292(1-2):73–75. doi: 10.1016/0014-5793(91)80837-s. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager R. I., Hendler R. W., Bose S. The ability of actinic light to modify the bacteriorhodopsin photocycle. Heterogeneity and/or photocooperativity? Eur J Biochem. 1995 May 1;229(3):589–595. doi: 10.1111/j.1432-1033.1995.tb20502.x. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Gerstein M., Oesterhelt D., Henderson R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 1993 Jan;12(1):1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Száraz S., Oesterhelt D., Ormos P. pH-induced structural changes in bacteriorhodopsin studied by Fourier transform infrared spectroscopy. Biophys J. 1994 Oct;67(4):1706–1712. doi: 10.1016/S0006-3495(94)80644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokaji Z. Cooperativity-regulated parallel pathways of the bacteriorhodopsin photocycle. FEBS Lett. 1995 Jan 3;357(2):156–160. doi: 10.1016/0014-5793(94)01344-z. [DOI] [PubMed] [Google Scholar]
- Tokaji Z. Dimeric-like kinetic cooperativity of the bacteriorhodopsin molecules in purple membranes. Biophys J. 1993 Sep;65(3):1130–1134. doi: 10.1016/S0006-3495(93)81165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Effects of hydrostatic pressure on the kinetics reveal a volume increase during the bacteriorhodopsin photocycle. Biochemistry. 1995 Sep 26;34(38):12161–12169. doi: 10.1021/bi00038a009. [DOI] [PubMed] [Google Scholar]
- Váró G., Zimányi L., Fan X., Sun L., Needleman R., Lanyi J. K. Photocycle of halorhodopsin from Halobacterium salinarium. Biophys J. 1995 May;68(5):2062–2072. doi: 10.1016/S0006-3495(95)80385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi L., Cao Y., Needleman R., Ottolenghi M., Lanyi J. K. Pathway of proton uptake in the bacteriorhodopsin photocycle. Biochemistry. 1993 Aug 3;32(30):7669–7678. doi: 10.1021/bi00081a010. [DOI] [PubMed] [Google Scholar]


