Figure 1.
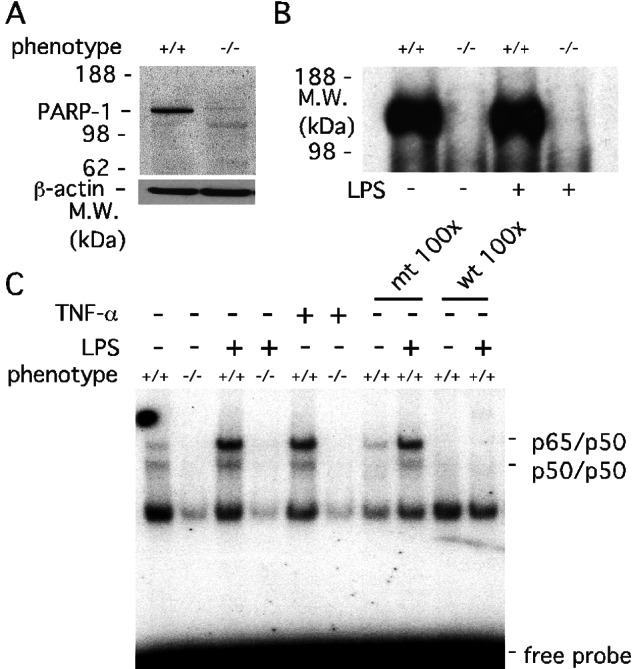
Activation of transcription factors is lost in PARP-1−/− primary mixed glial cells. (A) Protein immunoblot analysis of PARP-1 expression in PARP-1+/+ and PARP-1−/− glial cells. The PARP-1−/− lane confirmed the absence of PARP-1 protein. Total protein (50 μg) from PARP-1+/+ and PARP-1−/− glial cells was separated by 4–12% SDS/PAGE gel. β-actin was used as a protein loading control. M.W., molecular mass. (B) PARP-1 catalytic activity in PARP-1+/+ and PARP-1−/− glial cells. PARP-1 automodification by [32P]poly(ADP-ribose) was abolished in PARP-1−/− glial cells with or without LPS (1 μg/ml) for 30 min. Glial cells were labeled with [32P]NAD+ for 20 min at 4°C. Total protein (70 μg) from labeled glial cells was separated on 4–12% SDS/PAGE gel and visualized by autoradiography. (C) Defective NF-κB activation in PARP-1−/− glial cells. LPS- or TNF-α-induced DNA binding activity of NF-κB was absent in PARP-1−/− glial cells. Primary glial cells were treated with either 1 μg/ml LPS or 1 ng/ml (1,000 units/ml) TNF-α for 30 min, and the extracted nuclear fractions were analyzed for the DNA consensus binding activity of NF-κB heterodimers (p65/p50). Specificity of the DNA binding activity was assessed by using a 100-fold molar excess of unlabeled wild-type (wt) NF-κB oligonucleotides or single base mutated (mt) NF-κB oligonucleotides. Only wild type competed with labeled NF-κB oligonucleotides, indicating the specificity of the NF-κB DNA binding activity. The protein complex (p65/p50 and p50/p50 heterodimer) bound to the NF-κB element was confirmed by supershift using anti-p65 and anti-p50 antibodies (data not shown).
