Abstract
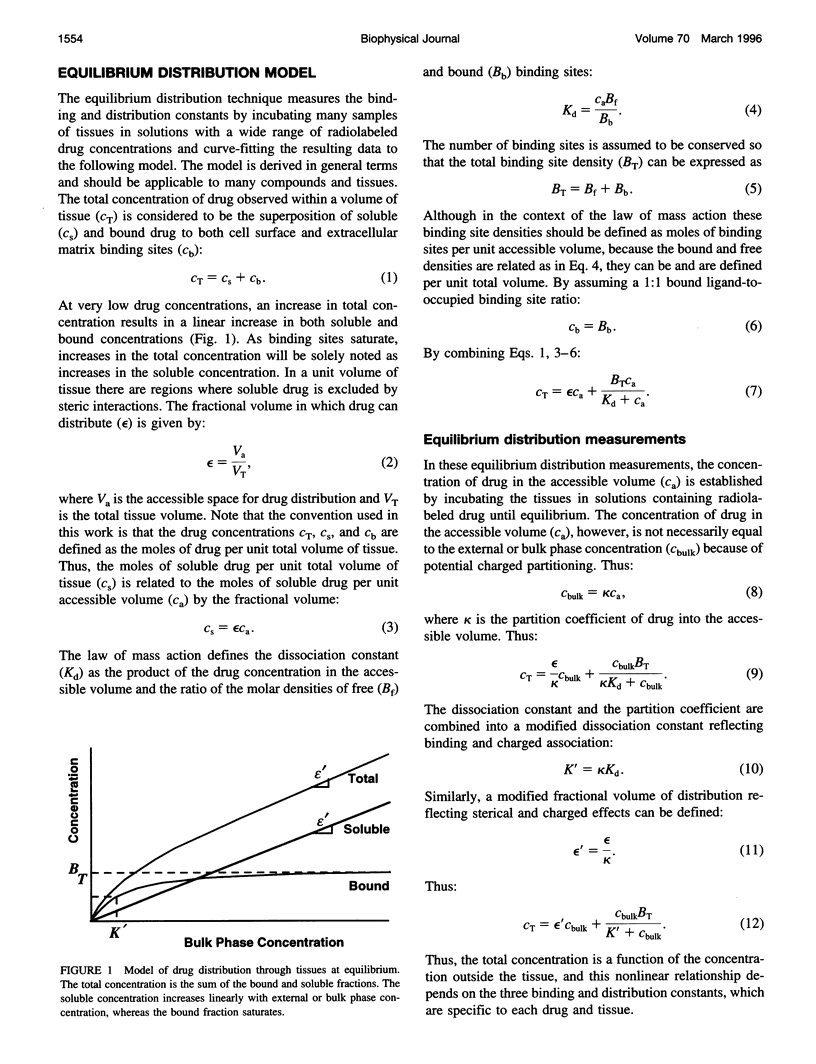
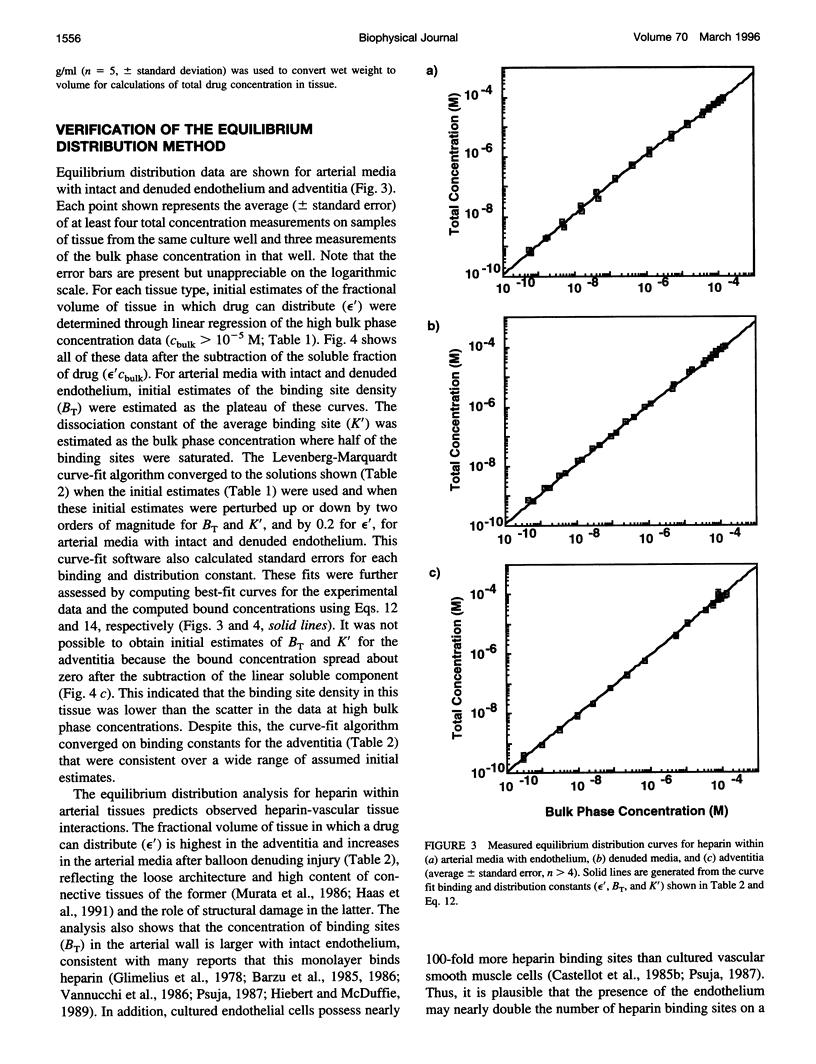
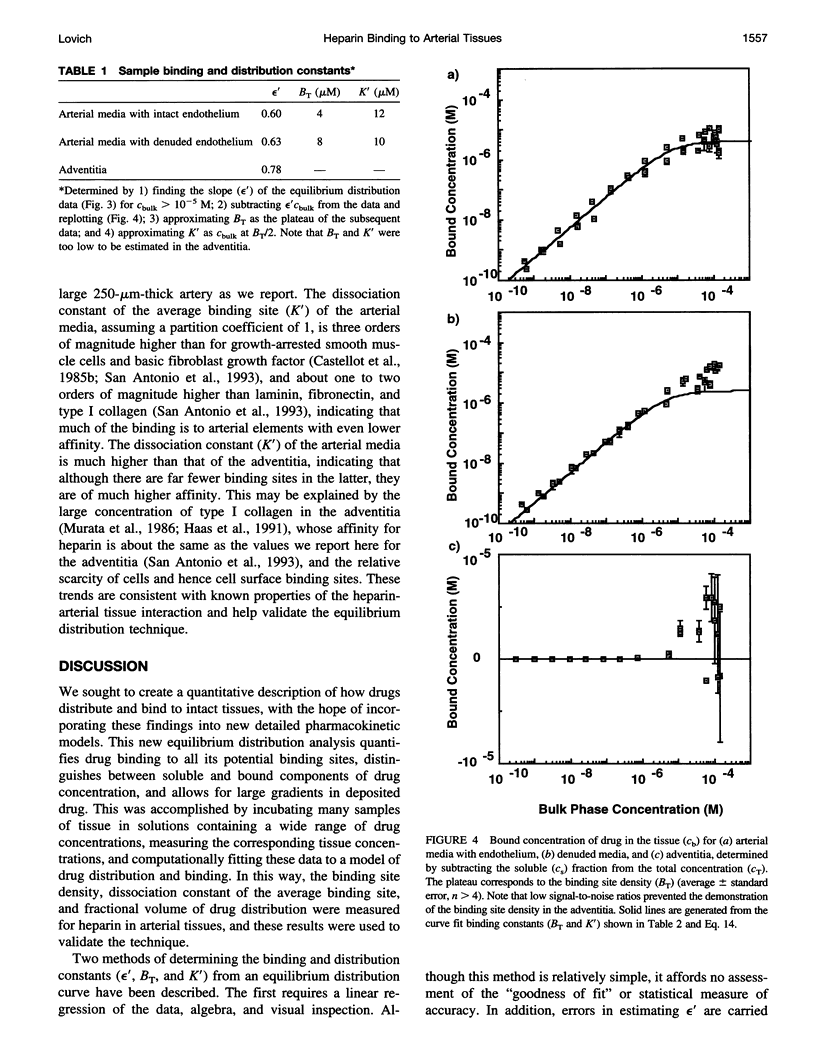
Classical pharmacokinetic descriptions do not adequately predict the dynamic and complex drug deposition patterns that follow some novel delivery techniques, in part because they do not characterize binding within intact tissues in sufficient detail. In this study, the binding site density of all the potential sites, the tissue-average dissociation constant, and the fractional volume in which heparin can distribute in arterial tissues were measured by incubating tissue samples to equilibrium in solutions containing a wide range of drug concentrations. An "equilibrium distribution curve" was constructed by plotting the concentration of drug in each sample against the concentration in the corresponding bulk phase. The above constants were determined by computationally fitting this curve to a model of drug distribution within tissues. The binding site density was measured to be 4.2 microM, 2.5 microM and 2.2 nM in porcine carotid media with intact and denuded endothelium, and adventitia, respectively. The dissociation constant of heparin in these tissues was estimated to be 6.8 microM, 5.0 microM, and 8.1 nM, respectively. The fractional tissue volume of distribution was 0.61, 0.70, and 0.87, respectively. These values are consistent with known properties of the heparin-arterial tissue interaction. Thus, this technique describes the cumulative effects of binding of a compound to all of its potential binding sites, and will be essential to new detailed descriptions of drug distribution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bârzu T., Molho P., Tobelem G., Petitou M., Caen J. Binding and endocytosis of heparin by human endothelial cells in culture. Biochim Biophys Acta. 1985 May 30;845(2):196–203. doi: 10.1016/0167-4889(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Bârzu T., Van Rijn J. L., Petitou M., Molho P., Tobelem G., Caen J. P. Endothelial binding sites for heparin. Specificity and role in heparin neutralization. Biochem J. 1986 Sep 15;238(3):847–854. doi: 10.1042/bj2380847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Cochran D. L., Karnovsky M. J. Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol. 1985 Jul;124(1):21–28. doi: 10.1002/jcp.1041240105. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Wong K., Herman B., Hoover R. L., Albertini D. F., Wright T. C., Caleb B. L., Karnovsky M. J. Binding and internalization of heparin by vascular smooth muscle cells. J Cell Physiol. 1985 Jul;124(1):13–20. doi: 10.1002/jcp.1041240104. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Wright T. C., Karnovsky M. J. Regulation of vascular smooth muscle cell growth by heparin and heparan sulfates. Semin Thromb Hemost. 1987 Oct;13(4):489–503. doi: 10.1055/s-2007-1003525. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Karnovsky M. J. Contrasting effects of the intermittent and continuous administration of heparin in experimental restenosis. Circulation. 1994 Feb;89(2):770–776. doi: 10.1161/01.cir.89.2.770. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Karnovsky M. J. Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1513–1517. doi: 10.1073/pnas.90.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. G., Roubin G. S., King S. B., 3rd, Douglas J. S., Jr, Weintraub W. S., Thomas R. G., Cox W. R. Angiographic and clinical predictors of acute closure after native vessel coronary angioplasty. Circulation. 1988 Feb;77(2):372–379. doi: 10.1161/01.cir.77.2.372. [DOI] [PubMed] [Google Scholar]
- Ellis S. G., Roubin G. S., Wilentz J., Douglas J. S., Jr, King S. B., 3rd Effect of 18- to 24-hour heparin administration for prevention of restenosis after uncomplicated coronary angioplasty. Am Heart J. 1989 Apr;117(4):777–782. doi: 10.1016/0002-8703(89)90612-1. [DOI] [PubMed] [Google Scholar]
- Glimelius B., Busch C., Hök M. Binding of heparin on the surface of cultured human endothelial cells. Thromb Res. 1978 May;12(5):773–782. doi: 10.1016/0049-3848(78)90271-2. [DOI] [PubMed] [Google Scholar]
- Haas K. S., Phillips S. J., Comerota A. J., White J. V. The architecture of adventitial elastin in the canine infrarenal aorta. Anat Rec. 1991 May;230(1):86–96. doi: 10.1002/ar.1092300109. [DOI] [PubMed] [Google Scholar]
- Hiebert L. M., McDuffie N. M. The intracellular uptake and protracted release of exogenous heparins by cultured endothelial cells. Artery. 1989;16(4):208–222. [PubMed] [Google Scholar]
- Ip J. H., Fuster V., Badimon L., Badimon J., Taubman M. B., Chesebro J. H. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990 Jun;15(7):1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- Lovich M. A., Edelman E. R. Mechanisms of transmural heparin transport in the rat abdominal aorta after local vascular delivery. Circ Res. 1995 Dec;77(6):1143–1150. doi: 10.1161/01.res.77.6.1143. [DOI] [PubMed] [Google Scholar]
- Murata K., Motayama T., Kotake C. Collagen types in various layers of the human aorta and their changes with the atherosclerotic process. Atherosclerosis. 1986 Jun;60(3):251–262. doi: 10.1016/0021-9150(86)90172-3. [DOI] [PubMed] [Google Scholar]
- Nugent M. A., Edelman E. R. Kinetics of basic fibroblast growth factor binding to its receptor and heparan sulfate proteoglycan: a mechanism for cooperactivity. Biochemistry. 1992 Sep 22;31(37):8876–8883. doi: 10.1021/bi00152a026. [DOI] [PubMed] [Google Scholar]
- Psuja P. Affinity of binding of radiolabeled (125I) heparin and low molecular weight heparin fraction CY 222 to endothelium in culture. Folia Haematol Int Mag Klin Morphol Blutforsch. 1987;114(3):429–436. [PubMed] [Google Scholar]
- Rogers C., Karnovsky M. J., Edelman E. R. Inhibition of experimental neointimal hyperplasia and thrombosis depends on the type of vascular injury and the site of drug administration. Circulation. 1993 Sep;88(3):1215–1221. doi: 10.1161/01.cir.88.3.1215. [DOI] [PubMed] [Google Scholar]
- San Antonio J. D., Slover J., Lawler J., Karnovsky M. J., Lander A. D. Specificity in the interactions of extracellular matrix proteins with subpopulations of the glycosaminoglycan heparin. Biochemistry. 1993 May 11;32(18):4746–4755. doi: 10.1021/bi00069a008. [DOI] [PubMed] [Google Scholar]
- Vannucchi S., Pasquali F., Chiarugi V., Ruggiero M. Internalization and metabolism of endogenous heparin by cultured endothelial cells. Biochem Biophys Res Commun. 1986 Oct 15;140(1):294–301. doi: 10.1016/0006-291x(86)91089-2. [DOI] [PubMed] [Google Scholar]


