Abstract
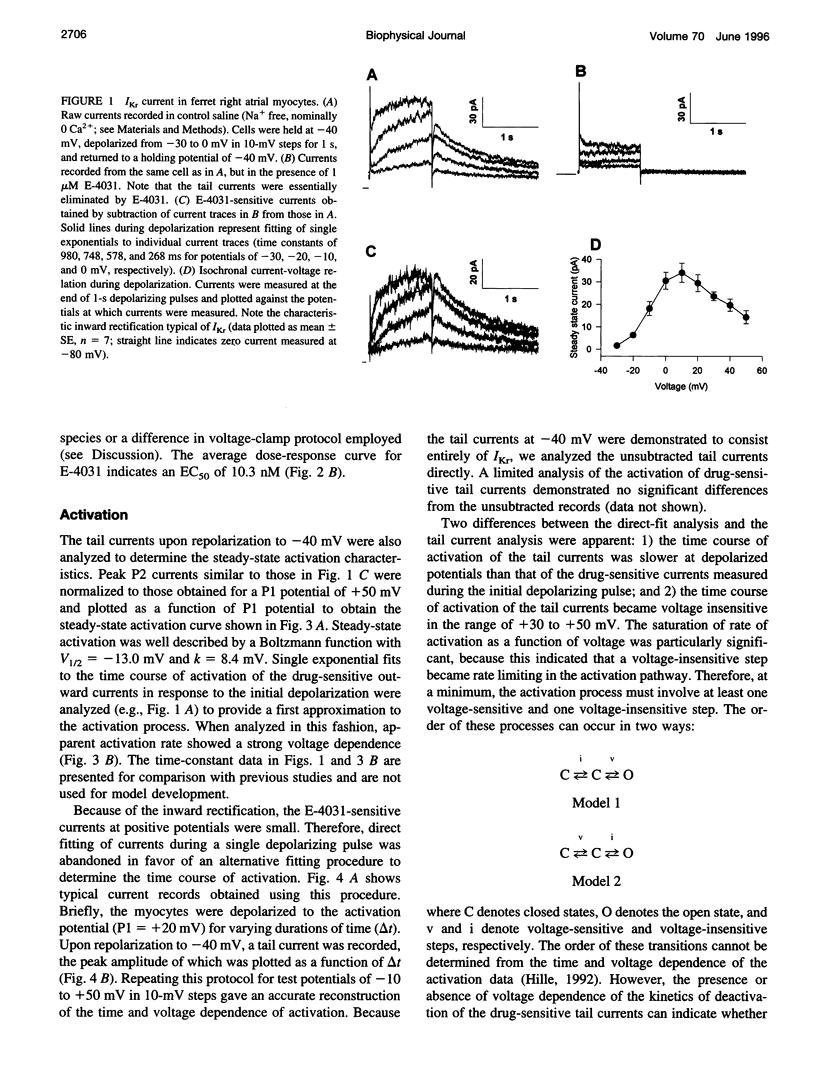
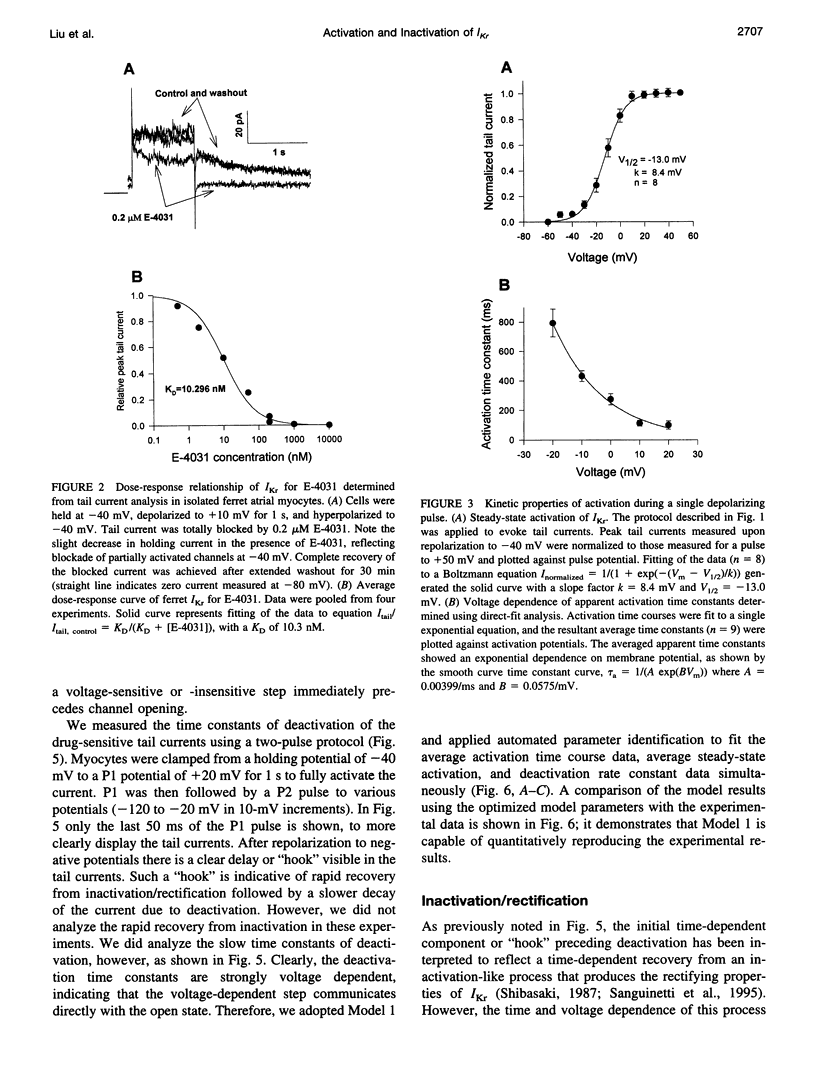
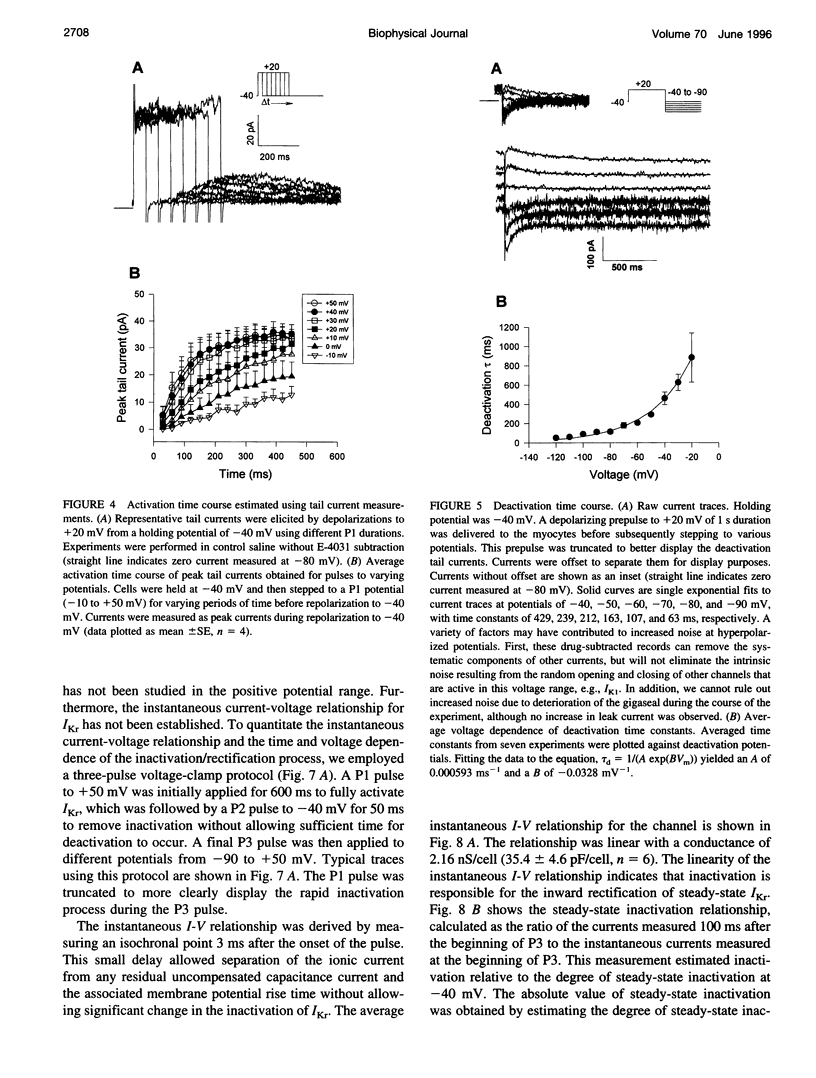
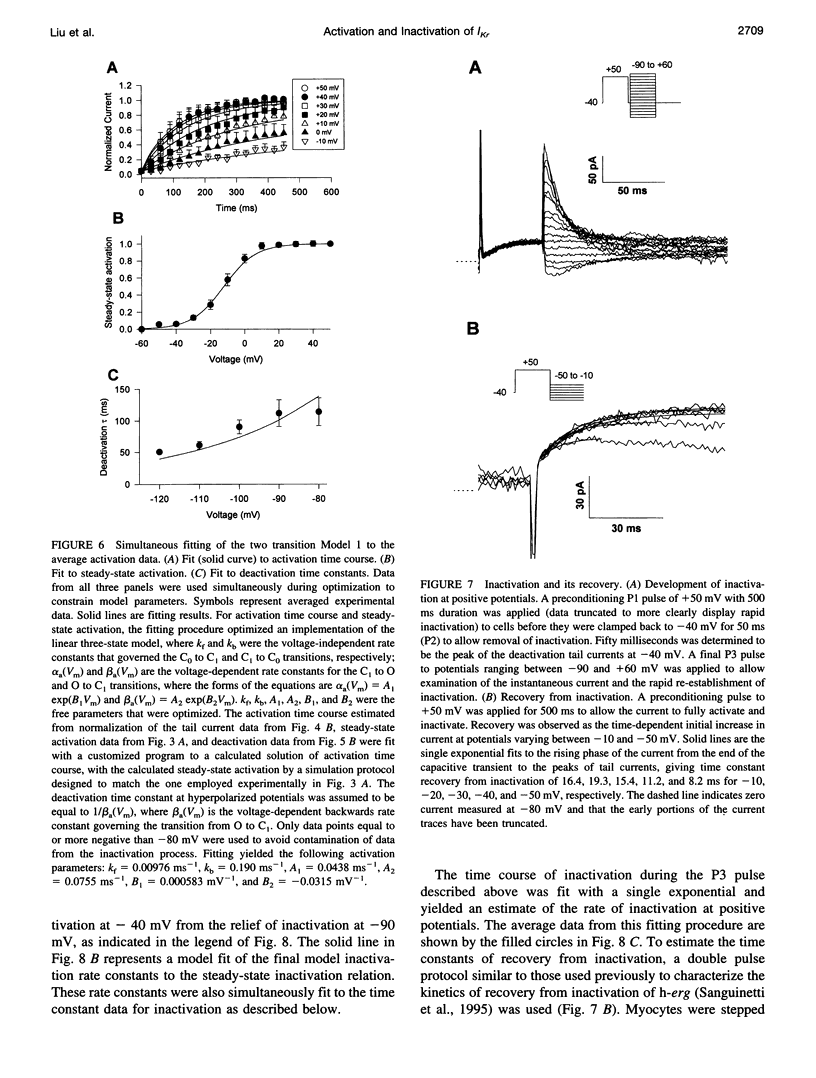
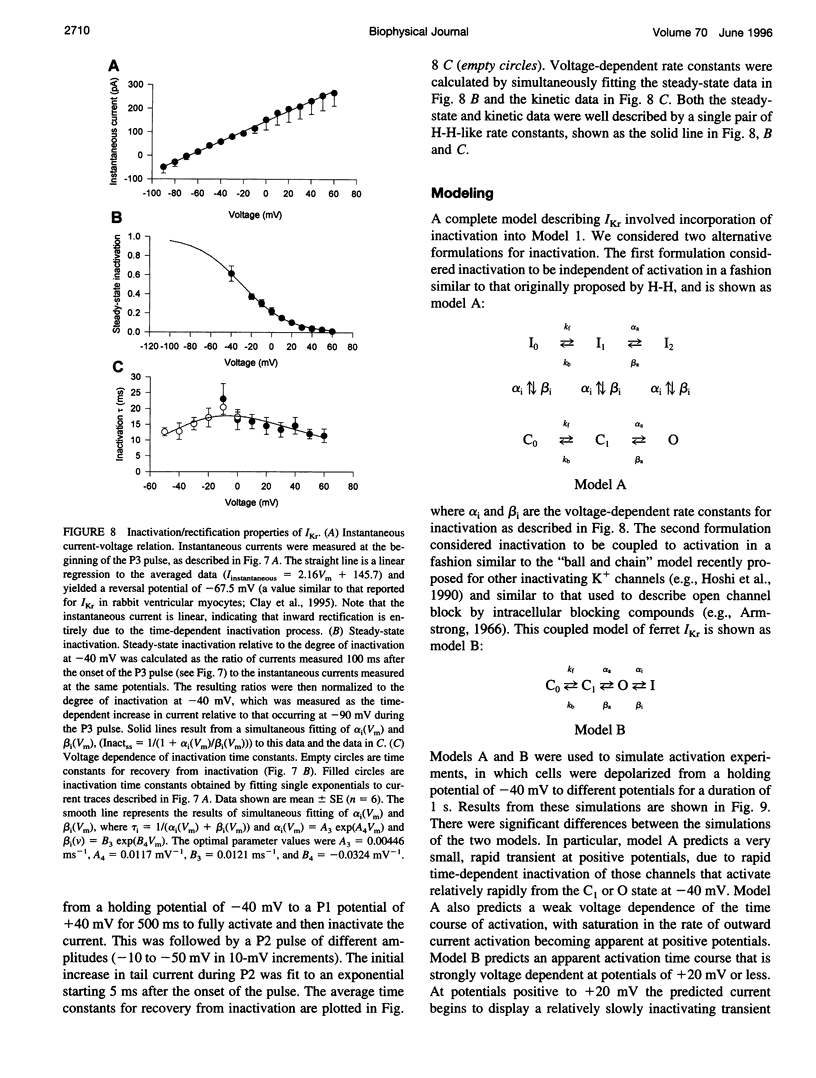
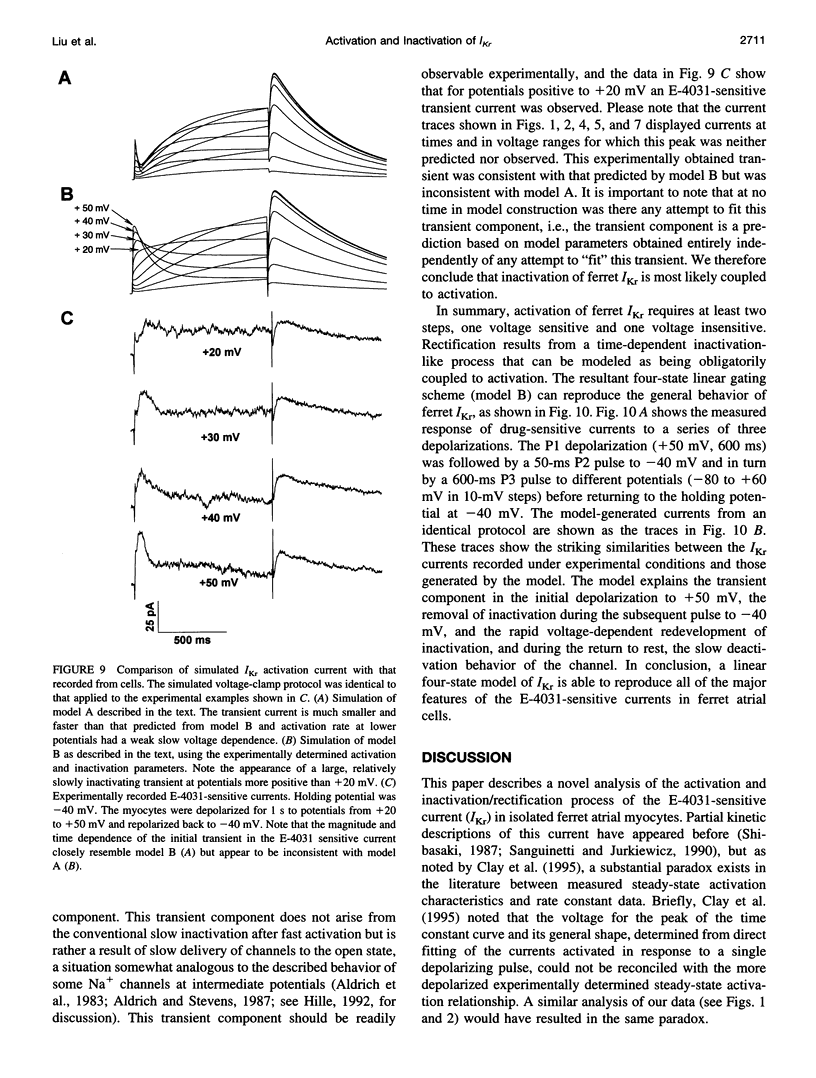
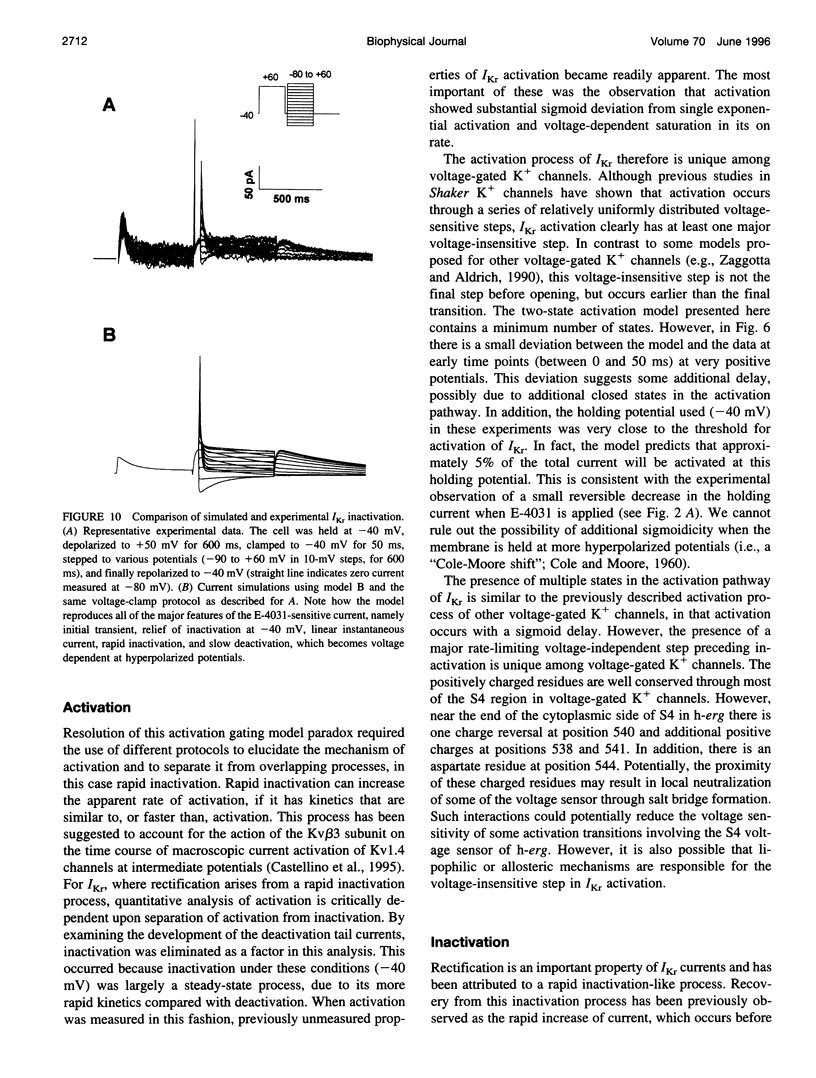
Ferret atrial myocytes can display an E-4031-sensitive current (IKr) that is similar to that previously described for guinea pig cardiac myocytes. We examined the ferret atrial IKr as the E-4031-sensitive component of current using the amphotericin B perforated patch-clamp technique. Steady-state IKr during depolarizing pulses showed characteristic inward rectification. Activation time constants during a single pulse were voltage dependent, consistent with previous studies. However, for potentials positive to +30 mV, IKr time course became complex and included a brief transient component. We examined the envelope of tails of the drug-sensitive current for activation in the range -10 to +50 mV and found that the tail currents for IKr do not activate with the same time course as the current during the depolarizing pulse. The activation time course determined from tail currents was relatively voltage insensitive over the range +30 to +50 mV (n = 5), but was voltage sensitive for potentials between -10 and +30 mV and appeared to show some sigmoidicity in this range. These data indicate that activation of IKr occurs in at least two steps, one voltage sensitive and one voltage insensitive, the latter of which becomes rate limiting at positive potentials. We also examined the rapid time-dependent inactivation process that mediates rectification at positive potentials. The time constants for this process were only weakly voltage dependent over the range of potentials from -50 to +60 mV. From these data we constructed a simple linear four-state model that reproduces the general features of ferret IKr, including the initial transient at positive potentials and the apparent discrepancy between the currents during the initial depolarizing pulse and the tail current.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987 Feb;7(2):418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumonwo J. M., Freeman L. C., Kwok W. M., Kass R. S. Delayed rectification in single cells isolated from guinea pig sinoatrial node. Am J Physiol. 1992 Mar;262(3 Pt 2):H921–H925. doi: 10.1152/ajpheart.1992.262.3.H921. [DOI] [PubMed] [Google Scholar]
- Apkon M., Nerbonne J. M. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J Gen Physiol. 1991 May;97(5):973–1011. doi: 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. doi: 10.1085/jgp.50.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx P. H., Marban E. Background potassium current active during the plateau of the action potential in guinea pig ventricular myocytes. Circ Res. 1993 Apr;72(4):890–900. doi: 10.1161/01.res.72.4.890. [DOI] [PubMed] [Google Scholar]
- Balser J. R., Bennett P. B., Roden D. M. Time-dependent outward current in guinea pig ventricular myocytes. Gating kinetics of the delayed rectifier. J Gen Physiol. 1990 Oct;96(4):835–863. doi: 10.1085/jgp.96.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993 Aug;73(2):379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Rasmusson R. L., Qu Y., Strauss H. C. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. I. Basic characterization and kinetic analysis. J Gen Physiol. 1993 Apr;101(4):571–601. doi: 10.1085/jgp.101.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Rasmusson R. L., Strauss H. C. Ionic current mechanisms generating vertebrate primary cardiac pacemaker activity at the single cell level: an integrative view. Annu Rev Physiol. 1992;54:279–302. doi: 10.1146/annurev.ph.54.030192.001431. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Use-dependent block and use-dependent unblock of the delayed rectifier K+ current by almokalant in rabbit ventricular myocytes. Circ Res. 1993 Nov;73(5):857–868. doi: 10.1161/01.res.73.5.857. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther. 1992 Aug;262(2):809–817. [PubMed] [Google Scholar]
- Castellino R. C., Morales M. J., Strauss H. C., Rasmusson R. L. Time- and voltage-dependent modulation of a Kv1.4 channel by a beta-subunit (Kv beta 3) cloned from ferret ventricle. Am J Physiol. 1995 Jul;269(1 Pt 2):H385–H391. doi: 10.1152/ajpheart.1995.269.1.H385. [DOI] [PubMed] [Google Scholar]
- Clay J. R., Ogbaghebriel A., Paquette T., Sasyniuk B. I., Shrier A. A quantitative description of the E-4031-sensitive repolarization current in rabbit ventricular myocytes. Biophys J. 1995 Nov;69(5):1830–1837. doi: 10.1016/S0006-3495(95)80053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Curran M. E., Splawski I., Timothy K. W., Vincent G. M., Green E. D., Keating M. T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995 Mar 10;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Fedida D., Wible B., Wang Z., Fermini B., Faust F., Nattel S., Brown A. M. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res. 1993 Jul;73(1):210–216. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Inoue M., Imanaga I. Masking of A-type K+ channel in guinea pig cardiac cells by extracellular Ca2+. Am J Physiol. 1993 Jun;264(6 Pt 1):C1434–C1438. doi: 10.1152/ajpcell.1993.264.6.C1434. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz N. K., Sanguinetti M. C. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993 Jan;72(1):75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki K., Imaizumi Y., Watanabe M., Habuchi Y., Giles W. R. Delayed rectifier K+ current in rabbit atrial myocytes. Am J Physiol. 1995 Aug;269(2 Pt 2):H524–H532. doi: 10.1152/ajpheart.1995.269.2.H524. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995 Apr 21;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. Am J Physiol. 1991 Feb;260(2 Pt 2):H393–H399. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Role of external Ca2+ and K+ in gating of cardiac delayed rectifier K+ currents. Pflugers Arch. 1992 Feb;420(2):180–186. doi: 10.1007/BF00374988. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E. F., Giles W. R. Ionic currents that generate the spontaneous diastolic depolarization in individual cardiac pacemaker cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7796–7800. doi: 10.1073/pnas.82.22.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyders D. J., Tamkun M. M., Bennett P. B. A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J Gen Physiol. 1993 Apr;101(4):513–543. doi: 10.1085/jgp.101.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995 Jul 7;269(5220):92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- Verheijck E. E., van Ginneken A. C., Bourier J., Bouman L. N. Effects of delayed rectifier current blockade by E-4031 on impulse generation in single sinoatrial nodal myocytes of the rabbit. Circ Res. 1995 Apr;76(4):607–615. doi: 10.1161/01.res.76.4.607. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993 Aug;73(2):276–285. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc Res. 1994 Oct;28(10):1540–1546. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Laurita K. R., Rosenbaum D. S., Rudy Y. Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formulation and their role in repolarization. Circ Res. 1995 Jul;77(1):140–152. doi: 10.1161/01.res.77.1.140. [DOI] [PubMed] [Google Scholar]


