Abstract
Silicon is essential for some plants, diatoms, and sponges but, in higher animals, its endogenous regulation has not been demonstrated. Silicate ions may be natural ligands for aluminum and here we show that, in the freshwater snail (Lymnaea stagnalis), intracellular silicon seems specifically up-regulated in response to sublethal aluminum exposure. X-ray microanalysis showed that exposure of snails to low levels of aluminum led to its accumulation in lysosomal granules, accompanied by marked up-regulation of silicon. Increased lysosomal levels of silicon were a specific response to aluminum because cadmium and zinc had no such effect. Furthermore, intra-lysosomal sulfur from metallothionein and other sulfur-containing ligands was increased after exposure to cadmium and zinc but not aluminum. To ensure that these findings indicated a specific in vivo response, and not ex vivo formation of hydroxy-aluminosilicates (HAS) from added aluminum (555 μg/liter) and water-borne silicon (43 μg/liter), two further studies were undertaken. In a ligand competition assay the lability of aluminum (527 μg/liter) was completely unaffected by the presence of silicon (46 μg/liter), suggesting the absence of HAS. In addition, exogenous silicon (6.5 mg/liter), added to the water column to promote formation of HAS, caused a decrease in lysosomal aluminum accumulation, showing that uptake of HAS would not explain the loading of aluminum into lysosomal granules. These findings, and arguments on the stability, lability, and kinetics of aluminum–silicate interactions, suggest that a silicon-specific mechanism exists for the in vivo detoxification of aluminum, which provides regulatory evidence of silicon in a multicellular organism.
The role of silicon (Si) in biological systems is poorly understood. It is essential in some plants (1) and some primitive organisms, such as algae (2) and sponges (3, 4), which utilize Si in their exoskeletons. In these organisms, the in vivo process of biosilicification involves controlled polymerization of silica (2) that is directed by specific protein filaments (3, 4) or cationic polypeptides (5, 6). An essential role for Si in bone and connective tissue formation in higher animals also has been proposed (7–9), but similar regulatory mechanisms have not been shown. Silicon, in the form of orthosilicic acid [monomeric silicic acid; Si(OH)4], interacts with the alkyl 1,4-diol groups of sugar residues (10) and also with polyhydroxy aluminum (Al), forming hydroxy-aluminosilicate polymers (HAS; ref. 11) and ameliorating Al toxicity (12). Hence, both Al-independent (7) and Al-dependent mechanisms (13) have been proposed for the in vivo action of Si. Presently, only exogenous Si is considered to prevent Al toxicity in animals, as regulation of endogenous Si, in response to Al exposure, has not been demonstrated. Indeed the metabolism of Si is thought to occur only in specific primitive organisms.
At neutral pH, environmental concentrations of polyhydroxy Al are bioavailable to grazing macroinvertebrates such as the freshwater snail, Lymnaea stagnalis (14, 15). Waterborne Al associates with secreted mucus films of L. stagnalis and is taken up by the mollusc during subsequent grazing (15). Exposure of L. stagnalis to elevated concentrations of metal ions, including Al, results in their detoxification by sequestration in specific lysosomal granules of digestive cells in the digestive gland (16, 17). During the exposure-detoxification process with Al, digestive cell and lysosomal morphology remain unchanged (16), and yet, L. stagnalis exhibits rapid behavioral suppression followed by tolerance (behavioral normalization) at around days 7−14, despite continued exposure to Al (18, 19). The initial behavioral-suppression stage can be overcome by adding orthosilicic acid (15:1, Si:Al) into the water column (18), which suggests that Al–Si interactions may be important in determining L. stagnalis responses during Al exposure. Here, we show that when L. stagnalis is exposed to Al alone, it accumulates the metal in lysosomal granules of digestive gland cells and, concomitantly, seems to up-regulate lysosomal levels of endogenous Si, providing a potential mechanism for enhancing detoxification of Al3+ (18).
Materials and Methods
Materials.
All solutions were prepared by using ultra-high purity (UHP) water, 18 MΩ/cm, from an Elga (High Wycombe, UK) water purifier. Polypropylene plastic ware (Nalgene; Fisher Scientific), acid washed [10% (vol/vol) HNO3] for 24 h and rinsed with UHP water, was used throughout. All chemicals were high purity (Merck or Aldrich Chem, unless otherwise indicated).
Experiment.
Lymnaea stagnalis (Sciento, Salford, UK), 3.5–5.6 g each and acclimatized to standard snail water (SSW: 222 mg/liter CaCl2/9.6 mg/liter MgSO4/4 mg/liter KHCO3/5.1 mg/liter KNO3/58 mg/liter NaHCO3; ref. 20), were exposed to either SSW alone‖ with no added metals, or to SSW with Al (500 μg/liter), zinc (Zn, 100 μg/liter), or cadmium (Cd, 50 μg/liter) in the presence and absence of orthosilicic acid (13:1, Si:M; M = Al, Zn, or Cd) for a period of 30 days. However, the water column was renewed every 2 days. Briefly, every 48 h, snails were removed from their exposure tank, rinsed in UHP water and placed in a second identical tank containing freshly prepared SSW (20 liters; with or without added orthosilicic acid) that had been aerated for 2 h. In tanks where orthosilicic acid was added, it was prepared by diluting concentrated, basic sodium silicate (7 M Si) in 20 liters SSW with neutralization to pH 7.3 and equilibration for 48 h. This preparation ensures that silicic acid is present in solution in a monomeric form (i.e., orthosilicic acid; refs. 21 and 22). Metal ions then were added to appropriate tanks at the concentrations noted above, from stock acidic solutions (pH < 3) prepared from the nitrate salts in SSW. Snails were sampled to determine Si concentrations of the digestive gland and remaining soft tissues as well as metal ion concentrations of lysosomal granules of the digestive gland cells (see below). Experiments were conducted in a cold room (at 8°C), and snails were kept under a 12 h light/12 h dark regime. The pH of the water column was monitored and maintained between 7.3–7.5 by the addition of HCl or NaOH. Snails were fed 10 g of lettuce per day for each group. Tanks were polypropylene, acid washed and thoroughly rinsed in UHP water before use. Following renewal of the water column every 48 h, tanks were thoroughly rinsed in UHP water before use.
Intracellular Levels of Metal Ions in Lysosomal Granules.
Digestive gland tissue from snails exposed to SSW without added metals (control) or from snails exposed to sublethal concentrations of the metals for 30 days were prepared for transmission electron microscopy (TEM) and x-ray microanalysis (XRMA), as described (16). Intracellular levels of Si, Al, Zn, Cd, and S were determined in the lysosomal granules within the digestive gland cells (Fig. 1). Sections were analyzed with a Philips 400 TEM (Philips, Eindhoven, The Netherlands) and x-rays were collected by using a KEVEX detector (Oxford Instruments; High Wycombe, UK). The x-ray spectra were visualized by using a Link 860 series II x-ray microanalytical system (Oxford Instruments). Elements were expressed as peak-to-background ratio (PBR) calculated with ISIS x-ray analysis software (v.1993, Oxford Instruments).
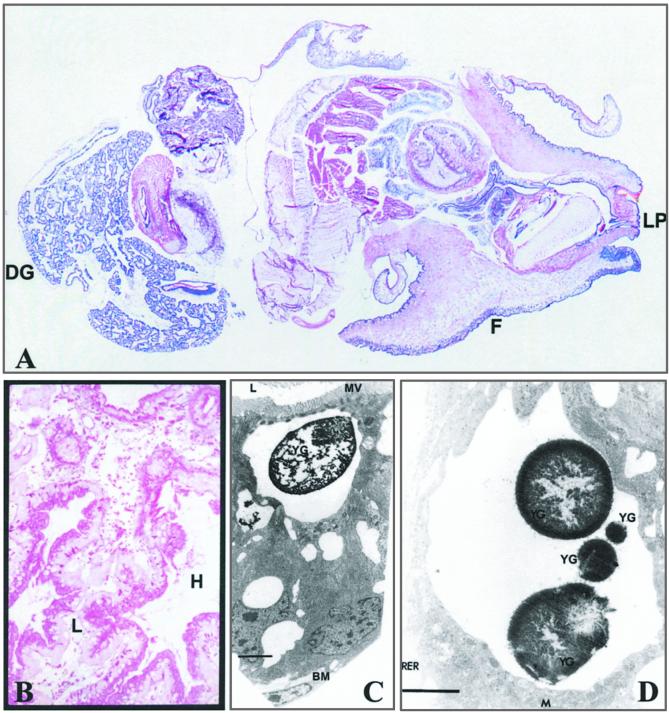
Figure 1.
(A) Longitudinal section of whole L. stagnalis stained with hemotoxylin blue. For orientation, the digestive gland (DG), foot (F), and lip (LP) are labeled. Scale is 4.2:1. (B) Higher-power (original × 100) section through the digestive gland stained with alcian blue at pH 2.5 followed by periodic acid Schiff. Digestive gland cells are shown with their apical aspect adjacent to the lumen (L), which contains cell debris, and their basolateral aspect (stained pink) adjacent to the haemolymph (H). (C) An example of a digestive cell of the digestive gland (ref. 16) at the excretory stage is shown by TEM. A yellow granule (YG) is shown; it can be identified by its large size and the vacuolated state of the lysosome. The basement membrane (BM), lumen (L), and microvilli brush border (MV) are labeled for orientation. (Bar = 2.5 μm.) (D) High-power TEM of a different vacuolated lysosome of a digestive cell with four yellow granules (YG). Mitochondria (M) and rough endoplasmic reticulum (RER) are labeled for orientation. (Bar = 2 μm.) XRMA was undertaken with a fixed electron beam spot size (360 nm) and magnification (×17,000) from electron-dense areas of granules.
Accumulation of Metal Ions Within Soft Tissues.
Total level of Si in whole digestive gland and remaining soft tissue was measured after the 30-day exposure period. Briefly, snails were removed, rinsed in UHP water, and rapidly killed by freezing with liquid nitrogen. After thawing, whole-snail tissue was separated from the shell, and the digestive gland was dissected away from the remaining tissue. The dissected samples were rinsed in UHP water and then oven-dried to a constant dry weight in individual acid-washed polypropylene containers and digested with 2 ml of 30% (wt/vol) H2O2 (AristaR) and 2 ml of 70% (wt/vol) HNO3 (Romil Pure Chemistry, Cambridge, UK). Digest blanks also were undertaken with UHP water in place of snail tissue. Digested samples were diluted with 12 ml of UHP water before elemental analysis (see below). Elemental concentrations were expressed per g of dry weight of tissue.
Elemental Analyses.
Snail-tissue digests were analyzed for Si by inductively coupled plasma optical emission spectrometry (JY24; Jobin-Yvon, Longjumeau, France) with a V-groove nebulizer and conventional Scott-type double-pass spray chamber at 251.611 nm. Peak profiles were used with a window size of 0.1 nm with 54 increments per profile and integration times of 0.1 s per increment. Sample flow rate was 1 ml/min. Samples were analyzed with spiked, sample-based standards, prepared by diluting 1,000 mg/liter Si standard solution into diluted tissue digests from snails.
Statistical Analysis.
Results are expressed as means ± SE. Comparisons between snails exposed to SSW without added metals (control) and snails exposed to sublethal concentrations of metals was done by one-way ANOVA, with significance taken as P < 0.05. A Bonferroni correction for multiplicity was applied to the P value where multiple comparisons were made (i.e., P/number of comparisons).
Results and Discussion
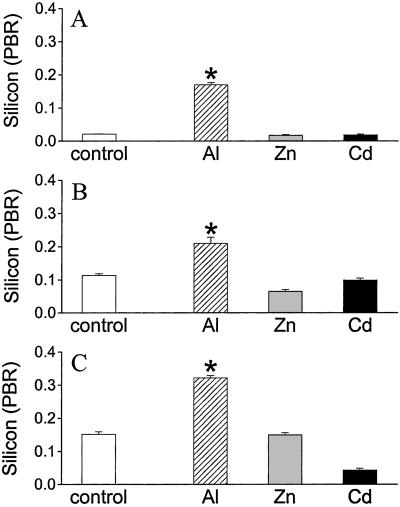
Inductively coupled plasma optical emission spectrometry showed that, in L. stagnalis, resting Si concentrations of the whole digestive gland (213 ± 36 μg/g dry weight) were twice that of the total remaining tissue and, by using TEM with XRMA, Si was found to be constitutively present in lysosomal granules of digestive gland cells (Fig. 2). Three granule types exist in these cells, namely small (diameter < 2 μm), green (<10 μm), and yellow granules (<13 μm) which, respectively, are thought to represent increasing states of maturity in the storage and excretion of lysosomal products (16, 17). Constitutive levels of Si in the lysosomal granules significantly increased through these phases of maturity [Fig. 2; P < 0.0001, green or yellow vs. small; P < 0.001, yellow vs. green (one-way ANOVA)]. After exposure of L. stagnalis to Al for 30 days (which is well into the period of behavioral normalization), there was a marked increase in Si levels in all three granule types in digestive gland cells (Fig. 2; P < 0.0001). Aluminum-induced levels of Si were, again, greatest in mature yellow granules, suggesting that Si was retained in a stable form and not reprocessed and absorbed as occurs for uncomplexed, lysosomal ligands (23).
Figure 2.
Silicon levels (peak-to-background ratio; PBR) of digestive cell granules from L. stagnalis exposed to SSW without added metal (control) or sublethal concentrations of Al (500 μg/liter), Zn (100 μg/liter), or Cd (50 μg/liter) for 30 days. Intracellular Si levels of small (A), green (B), and yellow (C) granules in digestive gland cells (digestive cells/excretory cells; refs. 16 and 17) are increased in L. stagnalis challenged with Al but not other metal ions. Intracellular measurements were by XRMA in tissue sections prepared for TEM. Values are means + SE (A–C, n = 100 granules per group). *, P < 0.0001 (one-way ANOVA), Al vs. control, for all granules. No significant increase for other metal ions vs. control.
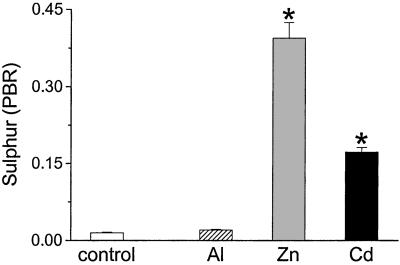
Zinc and Cd have high affinity for ligands with S as the donor atom and, as in mammals, are sequestered by snails in S-containing metallo-proteins (metallothioneins) of the cytoplasm before redistribution to the lysosomal granules (24). However, unlike Al, neither metal ion caused an increase in lysosomal Si levels (Fig. 2); in fact, there was some evidence for reduced Si levels in granules after exposure to Zn or Cd (Fig. 2). In contrast, and as expected, both Zn and Cd induced marked up-regulation of S in the mature granules by 26- and 11-fold, respectively (Fig. 3; P < 0.0001), whereas the increase in lysosomal S after exposure to Al was only 1.4-fold.
Figure 3.
Sulfur levels of yellow granules from digestive gland of L. stagnalis exposed to SSW without added metal (control) or sublethal concentrations of Al (500 μg/liter), Zn (100 μg/liter), or Cd (50 μg/liter) for 30 days. Sulfur was measured by XRMA under TEM and PBR was calculated. Values are means + SE (n = 100 granules per group). *, P < 0.0001 (one-way ANOVA), sulfur levels of yellow granules in L. stagnalis challenged with Al vs. those challenged with Zn or Cd.
These data demonstrate that lysosomal increases of Si in the cells of the digestive gland of L. stagnalis are specifically associated with the uptake and colocalization of Al. Because the x-ray energies of Al and Si are so close, PBR of XRMA results may be compared (Fig. 2, and see Fig. 5). The Si:Al ratio is ≈1:2.3, and it is likely that this ratio represents the presence of the nontoxic HAS, protoimogolite (Si:Al, 1:2–3; refs. 11, 25), the formation of which may explain the delayed but spontaneous behavioral normalization observed in L. stagnalis during Al exposure (18).
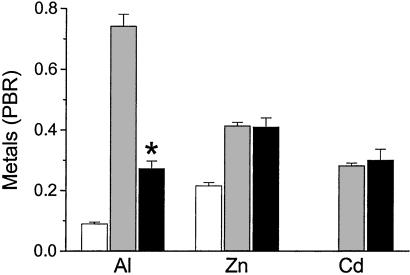
Figure 5.
Aluminum, Zn, and Cd levels in the yellow granules of digestive gland cells from L. stagnalis exposed for 30 days to SSW without added metal (control, white bars), or sublethal concentrations of Al (500 μg/liter), Zn (100 μg/liter), or Cd (50 μg/liter) in the absence (gray bars) and presence (black bars) of orthosilicic acid (Si:M molar ratio 13:1; M = Al, Zn or Cd). Metal PBR was obtained from XRMA and is shown as means + SE (n = 100 granules from two snails per group). *, P < 0.0001 (one-way ANOVA), effect of Si on Al loading into yellow granules vs. effect of Si on Cd and Zn levels.
A number of features suggest that Si is up-regulated at the cellular level in response to Al rather than combining with Al in the water column and then partitioning into the digestive gland as preformed HAS. First, there is a direct precedent for this mechanism, with Zn and Cd specifically up-regulating lysosomal ligands containing their preferred donor atom (S; ref. 24) in the same cell type of L. stagnalis (Fig. 3). Silicic acid would not be regulated at the transcriptional level, but it is possible that its lysosomal transport responds to Al loading. Second, Si was not added to the water column and was present only at 43 μg/liter as a contaminant compared with Al levels of 555 μg/liter (added + contaminant). At pH 7.3, and with this level of aquated Si, polyhydroxy Al formation precludes the growth of HAS (12, 25–30). Third, the reproducible pattern of Al toxicity in L. stagnalis, namely, behavioral changes at days 2–7 followed by tolerance at days 7–14 (ref. 18) without any changes to the experimental conditions, is consistent with delayed cellular responsiveness rather than environmental interactions. Indeed, the water column was renewed every 48 h, so even kinetic arguments of ex vivo HAS formation (11, 28, 30, 31) would not explain these observations on toxicity and tolerance.
To confirm that, under these conditions, Al and Si did not form HAS in the water column, with subsequent uptake by L. stagnalis, we undertook two further experiments.
Lability of Polyhydroxy Aluminum in the Presence and Absence of Orthosilicic Acid
Experiment.
Three polypropylene containers (Nalgene, Merck) were prepared with 250 ml of high-purity 5 mM MOPS buffer (minimum assay 99%, pH 7.36; Merck) in UHP water. Si at 46 μg/liter was added to one container and 5.5 mg/liter Si to another container from a stock basic sodium silicate solution (196 g/liter Si, pH 14; Aldrich). Following pH adjustment to 7.36, solutions were left to equilibrate at room temperature for 48 hr to ensure that only orthosilicic acid was present in solution. Al at 527 μg/liter (Al nitrate in UHP water, pH 3; minimum assay 99%; Merck) was then added to the three containers. An aliquot of each of the solutions was immediately removed to determine the lability of Al in these solutions (see below), and then further aliquots were taken at 6 hr, 24 hr, and 48 hr. Samples of the solutions also were collected at the different time points for total analysis of Al and Si in solution (see below).
Lability of Aluminium.
Lability of Al in solution was measured with the UV-active M3+ chelator, 1,2-dimethyl-3-hydroxy-4(1H)-pyridinone (DMHP), as described (15, 21). Free (i.e. unbound) DMHP absorbs strongly at 274 nm and is distinct from the Al bound chelator that absorbs at 289 nm. Briefly, 100 μl of 0.59 mM DMHP (high purity; Vitra Pharmaceutical, Clavering, UK) prepared in 5 mM MOPS buffer (pH 7.36) was added to a 2.9-ml aliquot of the Al-containing solution in a 1-cm quartz cell (Merck). The solution was immediately shaken and the absorbance at 274 nm measured over time to a constant value at 25°C (LKB Biochrom Ultraspec II Spectrophotometer, Pharmacia Biotech, UK).
Elemental Analyses.
Total Al and Si in solution was measured by inductively coupled plasma optical emission spectrometry (JY24, Jobin Yvon) at 396.152 and 251.611 nm, respectively. Integration times were 0.5 s. Samples were acidified to 1% (vol/vol) HNO3 (puriss p.a. plus; Aldrich) and analysed with standards prepared in 5 mM MOPS buffer acidified to 1% (vol/vol) HNO3 (puriss p.a. plus).
The Lability of Polyhydroxy Al.
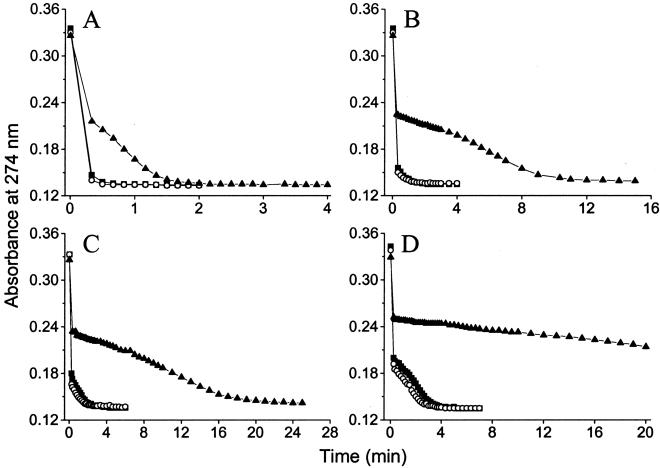
The lability of polyhydroxy Al in the presence and absence of orthosilicic acid was assessed by using 1,2-dimethyl-3-hydroxy-4(1H)-pyridinone (DMHP) which is sensitive to the presence of HAS (15, 21). To avoid contaminant Si, DMHP was added to UHP water buffered to pH 7.36 with or without added orthosilicic acid (46 μg/liter) at different time intervals from the addition of Al (527 μg/liter). Rates of Al/DMHP complexation were progressively slower between 1 min and 48 h, as expected, with the formation of polyhydroxy Al (15, 18, 28), but Si (46 μg/liter) had no further effect (Fig. 4). In contrast, 5.5 mg/liter orthosilicic acid (≈10:1, Si:Al), which promotes formation of HAS in the water column (11, 12, 25, 30), markedly altered the rate of Al/DMHP complexation** at all time points (Fig. 4).
Figure 4.
Lability of Al (527 μg/liter) at pH 7.36 within 1 min (A), 6 h (B), 24 h (C), and 48 h (D) of its addition to UHP water containing 5 mM Mops buffer alone (control, ▪), Mops buffer plus 46 μg/liter Si (○), and Mops buffer plus 5.5 mg/liter Si (▴). Aluminum lability was determined by measuring its rate of complexation with DMHP at 274 nm as a function of time. Free (i.e., unbound) DMHP absorbs strongly at 274 nm, which is distinct to Al-bound DMHP that absorbs strongly at 289 nm. A lower absorbance represents Al binding (i.e., quantity of Al bound), whereas the pattern of absorbance with time is dictated by the lability of different Al species in the water column. The pattern of Al binding is identical in the presence or absence of 46 μg/liter Si.
Exogenous Silicon and L. stagnalis.
Finally, we exposed L. stagnalis to Al and Si under conditions favorable to the formation of HAS (13:1, Si:Al) (Fig. 4 and refs. 11, 12, 25, and 30). Levels of Al actually fell in the yellow granules (Fig. 5), compared with Al-exposed animals without added Si, suggesting that lysosomal uptake of HAS would not explain our earlier data on Al accumulation. Interestingly, the influence of exogenous Si on the uptake of metal ions into the yellow granules was, again, specific to Al (Fig. 5); this finding may represent a separate effect of exogenous Si on overall Al uptake and requires further investigation.
It seems clear from these data that uptake of waterborne HAS does not explain the concomitant occurrence of Al and Si in digestive cell lysosomes of L. stagnalis. In contrast, up-regulation of intracellular Si in response to Al accumulation would explain these findings, although we also considered the possibility of ex vivo formation and then uptake of soluble aluminosilicate anions such as [(HO)3SiOAl(OH)3]− (11, 27, 33). This mechanism cannot be excluded from our data: the experimental conditions (low contaminant Si levels, fresh water column every 48 h) may even favor the formation of soluble aluminosilicate anions over polymeric HAS, and perhaps these could be preferentially partitioned into digestive gland cell lysosomes. However, any such species would need an Al:Si ratio of 2.3:1, be sufficiently labile to preclude detection by the DMHP assay, and yet be sufficiently stable to avoid degradation in the acidic gut and escape lysosomal processing (see above). Based upon current understanding of soluble aluminosilicates, which do have high lability but also have low stability under even mildly acidic conditions (11, 27, 33), this mechanism is unlikely and would require the advancement of a novel anion species. Moreover, as noted above, this would still not explain the pattern of Al toxicity in L. stagnalis (18), which is consistent with cellular responsiveness and not with changes in environmental chemistry. Thus, taken together, our data strongly favor specific regulation of endogenous Si by L. stagnalis in an Al-responsive fashion. Silicon is markedly up-regulated in mature, stable yellow granules of the digestive gland in response to Al loading which, we propose, may promote in situ formation of the nontoxic HAS, protoimogolite, as evidenced by lysosomal Si:Al ratios.
These findings are of broad significance. The codeposition of Si and Al (or other metal ions) has also been shown in higher plants and, similarly, suggested as a mechanism of detoxification (34, 35). Although much of the interaction occurs extracellularly, typically at the epidermal cell wall and intercellular space (34–38), intracellular deposition of Si has been reported, notably in an Al-tolerant maize variety (38). Aluminum and Si were codeposited in the vacuoles of the expanding root cortical cells after exposure to Al for 24 h (38). Equivalent vacuolar codeposition of Al and Si in the human brain with Alzheimer's disease is suggested by data on the inorganic content of isolated lipofuscin granules, which are lysosomal degradation products (39, 40). It is possible, therefore, that vacuolar/lysosomal codeposition of Si and Al is a conserved mechanism in plants and animals, which, based upon the findings here, would represent a specific mechanism for detoxification of Al through the up-regulation of intracellular Si. Moreover, these data may provide a significant indication of the biological regulation of Si in a multicellular organism.
Acknowledgments
We thank the University of Manchester School of Biological Sciences Electron Microscopy Unit and Mr. Stefan Buk at St. Thomas' Hospital (Department of Histopathology) for technical assistance. We also acknowledge the reviewers and the editor for their critique and their help in advancing the academic discussion of the manuscript. This work was supported by the Special Trustees of St. Thomas' Hospital, The Wellcome Trust, and the Government of Egypt.
Abbreviations
- HAS
hydroxy-aluminosilicate polymers
- UHP
ultra-high purity
- SSW
standard snail water
- TEM
transmission electron microscopy
- XRMA
x-ray microanalysis
- DMHP
1,2-dimethyl-3-hydroxy-4(1H)-pyridinone
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
SSW contained traces of Si, Al, and Zn [43 ± 10, 55 ± 7, and 13.7 ± 4 μg/liter (means ± SE), respectively], mainly from the salts used to prepare SSW.
It should be noted that previous work (21) showing no effect of orthosilicic acid on Al/DMHP complexation considered monomeric Al (Al3+) and not polyhydroxy Al. Unlike oligomeric silicic acid, orthosilicic acid has weak affinity for the former (21, 27) but interacts significantly with the latter (11, 28, 32).
References
- 1.Epstein E. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrand M, Volcani B E, Gassman W, Schroeder J I. Nature (London) 1997;385:688–689. doi: 10.1038/385688b0. [DOI] [PubMed] [Google Scholar]
- 3.Cha J N, Shimizu K, Zhou Y, Christiansen S C, Chmelka B F, Stucky G D, Morse D E. Proc Natl Acad Sci USA. 1999;96:361–365. doi: 10.1073/pnas.96.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu K, Cha J, Stucky G D, Morse D E. Proc Natl Acad Sci USA. 1998;95:6234–6238. doi: 10.1073/pnas.95.11.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kröger N, Deutzmann R, Sumper M. Science. 1999;286:1129–1132. doi: 10.1126/science.286.5442.1129. [DOI] [PubMed] [Google Scholar]
- 6.Kröger N, Deutzmann R, Bergsdorf C, Sumper M. Proc Natl Acad Sci USA. 2000;97:14133–14138. doi: 10.1073/pnas.260496497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlisle E M. In: Silicon Biochemistry. Evered D, O'Connor M, editors. Chichester, U.K.: John Wiley & Sons; 1986. pp. 123–139. [Google Scholar]
- 8.Schwarz K, Milne D B. Nature (London) 1972;239:333–334. doi: 10.1038/239333a0. [DOI] [PubMed] [Google Scholar]
- 9.Carlisle E M. Science. 1972;178:619–621. doi: 10.1126/science.178.4061.619. [DOI] [PubMed] [Google Scholar]
- 10.Kinrade S D, Del Nin J W, Schach A S, Sloan T A, Wilson K L, Knight T G. Science. 1999;285:1542–1545. doi: 10.1126/science.285.5433.1542. [DOI] [PubMed] [Google Scholar]
- 11.Doucet F, Schneider C, Bones S J, Kretchmer A, Moss I, Tekely P, Exley C. Geochem Cosmochim Acta. 2001;65:2461–2467. [Google Scholar]
- 12.Birchall J D, Exley C, Chappell J S, Phillips M J. Nature (London) 1989;338:146–148. [Google Scholar]
- 13.Birchall J D. Chem Soc Rev. 1995;24:351–357. [Google Scholar]
- 14.Elangovan R, White K N, McCrohan C R. Environ Poll. 1997;96:29–33. doi: 10.1016/s0269-7491(97)00009-2. [DOI] [PubMed] [Google Scholar]
- 15.Jugdaohsingh R, Campbell M M, Thompson R P H, McCrohan C R, White K N, Powell J J. Environ Sci Technol. 1998;32:2591–2595. [Google Scholar]
- 16.Elangovan R, Powell J J, McCrohan C R, Ballance S, White K N. Tissue Cell. 2000;32:79–87. doi: 10.1054/tice.1999.0089. [DOI] [PubMed] [Google Scholar]
- 17.Brooks A W, White K N. Tissue Cell. 1995;27:61–72. doi: 10.1016/s0040-8166(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 18.Campbell M M, White K N, Jugdaohsingh R, Powell J J, McCrohan C R. Can J Fish Aquat Sci. 2000;57:1151–1159. [Google Scholar]
- 19.Campbell M M, Jugdaohsingh R, White K N, Powell J J, McCrohan C R. J Toxicol Environ Health. 2000;59A:253–270. doi: 10.1080/009841000156925. [DOI] [PubMed] [Google Scholar]
- 20.Thomas R, Lough A S, Lodge R W J. Appl Ecol. 1975;12:421–436. [Google Scholar]
- 21.Taylor P D, Jugdaohsingh R, Powell J J. J Am Chem Soc. 1997;119:8852–8856. [Google Scholar]
- 22.Jugdaohsingh R, Reffitt D M, Oldham C, Day J P, Fifield K, Thompson R P H, Powell J J. Am J Clin Nutr. 2000;71:944–949. doi: 10.1093/ajcn/71.4.944. [DOI] [PubMed] [Google Scholar]
- 23.Hopkin S P. Ecophysiology of Metals in Terrestrial Invertebrates. London: Elsevier Science; 1989. [Google Scholar]
- 24.Dallinger R, Berger B, Hunziker P, Kagi J H R. Nature (London) 1997;388:237–238. doi: 10.1038/40785. [DOI] [PubMed] [Google Scholar]
- 25.Lumsdon D G, Farmer V C. Eur J Soil Sci. 1995;46:179–186. [Google Scholar]
- 26.Farmer V C, Lumsdon D G. Geochem Cosmochim Acta. 1995;59:1019. [Google Scholar]
- 27.Farmer V C, Lumsdon D G. Geochem Cosmochim Acta. 1994;58:3331–3334. [Google Scholar]
- 28.Exley C, Birchall J D. Polyhedron. 1993;12:1007–1017. [Google Scholar]
- 29.Exley C, Birchall J D. Polyhedron. 1992;11:1901–1907. [Google Scholar]
- 30.Exley C, Pinnegar J K, Taylor H. J Theor Biol. 1997;189:133–139. doi: 10.1006/jtbi.1997.0501. [DOI] [PubMed] [Google Scholar]
- 31.Wada S-I, Wada K. J Soil Sci. 1980;31:457–467. [Google Scholar]
- 32.Duan J, Gregory J. J Inorg Biochem. 1998;69:193–201. [Google Scholar]
- 33.North M R, Fleischer M A, Swaddle T W. Can J Chem. 2001;79:75–79. [Google Scholar]
- 34.Wang L, Wang Y, Chen Q, Cao W, Li M, Zhang F. J Plant Nutr. 2000;23:1397–1406. [Google Scholar]
- 35.Neuman D, Nieden U Z. Phytochemistry. 2001;56:685–692. doi: 10.1016/s0031-9422(00)00472-6. [DOI] [PubMed] [Google Scholar]
- 36.Hammond K E, Evans D E, Hodson M J. Plant and Soil. 1995;173:89–95. [Google Scholar]
- 37.Hodson M J, Evans D E. J Exp Bot. 1995;46:161–171. [Google Scholar]
- 38.Hodson M J, Sangster A G. J Inorg Biochem. 1999;76:89–98. [Google Scholar]
- 39.Tokutake S, Nagase H, Morisaki S, Oyanagi S. Neurosci Lett. 1995;185:99–102. doi: 10.1016/0304-3940(94)11234-a. [DOI] [PubMed] [Google Scholar]
- 40.Tokutake S. Ann NY Acad Sci. 1997;826:510–512. doi: 10.1111/j.1749-6632.1997.tb48515.x. [DOI] [PubMed] [Google Scholar]