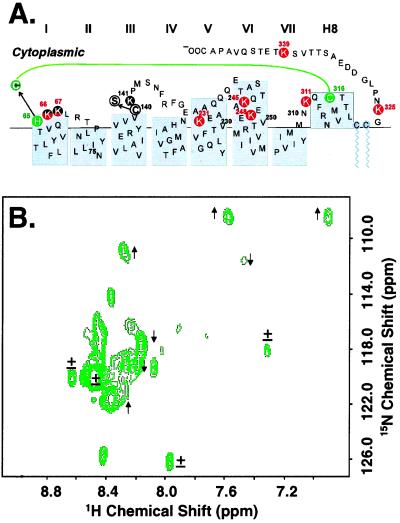
Figure 4.
(A) Secondary structure of the CP portion of a bovine rhodopsin mutant with a disulfide cross link between Cys-65 and Cys-316 (green). The mutant contains the engineered H65C and C140S mutations. (B) Two-dimensional HSQC spectrum of [α-15N]Lys-rhodopsin mutant shown in A. The spectrum was recorded at 750 MHz at 37°C. Indicated by arrows are signals that show increase (↑) or decrease (↓) in line width when compared with the WT (Fig. 3); ± indicates no change.