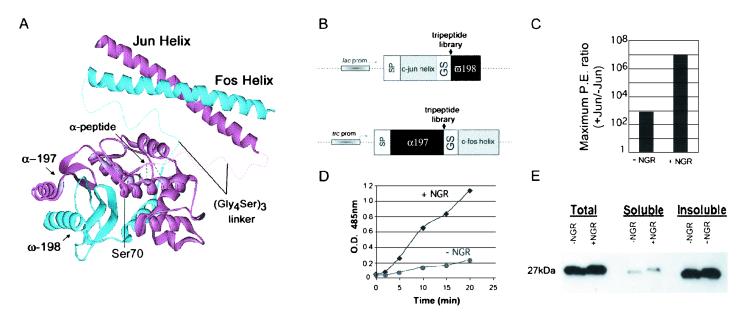
Figure 1.
β-Lactamase fragment structure and complementation assays in bacteria. (A) Structure of TEM-1 β-lactamase and illustrations of its reconstitution from the α197 (amino acids 25–197) and ω198 (amino acids 198–288) fragments connected by the flexible linker (Gly4Ser)3 and brought into contact by dimerization of the Fos and Jun helices. Placement of the α-tripeptide library is labeled with possible contacts as well as the active site serine (Ser-70). (B) Vectors used for expression of β-lactamase fragment fusion proteins with random tripeptide libraries inserted at the fragmentation point. Also indicated are signal peptide (SP), lactose operon promoter (lac prom), fusion of the tryptophan operon, lactose operon promoters (trc prom), and the glycine-serine linker (GS) as described for A. (C) Cells expressing the complementing constructs, α197Jun and ω198Fos, were plated on a range of ampicillin concentrations (10–200 μg/ml). The number of colonies obtained (signal) was divided by the number of colonies derived from plating the same constructs without the Jun helix (background) on the corresponding ampicillin concentration. The same assay was performed with the α197 fragment containing the NGR peptide insertion. The largest ratio obtained for each was designated as the maximum plating efficiency (P.E.) ratio, which is representative of five independent experiments. (D) Total β-lactamase activity was determined by assaying cells expressing the α197Jun and ω198Fos with and without the NGR insertion. The cells were induced to express the fusion proteins and assayed at equivalent cell densities in excess substrate (nitrocefin). Absorbance at 485 nM was determined in the cell-free supernatants of each sample and plotted against time. (E) To determine the effect of the NGR peptide on the stability of the β-lactamase α197 fragment, cells (from D) at 2 h of induction were lysed, and the accumulated protein was immunoblotted by using an antibody to the flag-tagged α197 fragment.