Abstract
The structures of the actin and myosin filaments of striated muscle have been studied extensively in the past by sectioning of fixed specimens. However, chemical fixation alters molecular details and prevents biochemically induced structural changes. To overcome these problems, we investigate here the potential of cryosectioning unfixed muscle. In cryosections of relaxed, unfixed specimens, individual myosin filaments displayed the characteristic helical organization of detached cross-bridges, but the filament lattice had disintegrated. To preserve both the filament lattice and the molecular structure of the filaments, we decided to section unfixed rigor muscle, stabilized by actomyosin cross-bridges. The best sections showed periodic, angled cross-bridges attached to actin and their Fourier transforms displayed layer lines similar to those in x-ray diffraction patterns of rigor muscle. To preserve relaxed filaments in their original lattice, unfixed sections of rigor muscle were picked up on a grid and relaxed before negative staining. The myosin and actin filaments showed the characteristic helical arrangements of detached cross-bridges and actin subunits, and Fourier transforms were similar to x-ray patterns of relaxed muscle. We conclude that the rigor structure of muscle and the ability of the filament lattice to undergo the rigor-relaxed transformation can be preserved in unfixed cryosections. In the future, it should be possible to carry out dynamic studies of active sacromeres by cryo-electron microscopy.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig R., Alamo L., Padrón R. Structure of the myosin filaments of relaxed and rigor vertebrate striated muscle studied by rapid freezing electron microscopy. J Mol Biol. 1992 Nov 20;228(2):474–487. doi: 10.1016/0022-2836(92)90836-9. [DOI] [PubMed] [Google Scholar]
- Frado L. L., Craig R. Electron microscopy of the actin-myosin head complex in the presence of ATP. J Mol Biol. 1992 Jan 20;223(2):391–397. doi: 10.1016/0022-2836(92)90659-8. [DOI] [PubMed] [Google Scholar]
- Fukami A., Adachi K. A new method of preparation of a self-perforated micro plastic grid and its application. J Electron Microsc (Tokyo) 1965;14(2):112–118. [PubMed] [Google Scholar]
- Hirose K., Lenart T. D., Murray J. M., Franzini-Armstrong C., Goldman Y. E. Flash and smash: rapid freezing of muscle fibers activated by photolysis of caged ATP. Biophys J. 1993 Jul;65(1):397–408. doi: 10.1016/S0006-3495(93)81061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Levine R. J. An electron microscopic and optical diffraction analysis of the structure of Limulus telson muscle thick filaments. J Cell Biol. 1982 Feb;92(2):443–451. doi: 10.1083/jcb.92.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Levine R. J., Stewart M. Electron microscopic and optical diffraction analysis of the structure of scorpion muscle thick filaments. J Cell Biol. 1985 Aug;101(2):395–401. doi: 10.1083/jcb.101.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepault J., Erk I., Nicolas G., Ranck J. L. Time-resolved cryo-electron microscopy of vitrified muscular components. J Microsc. 1991 Jan;161(Pt 1):47–57. doi: 10.1111/j.1365-2818.1991.tb03072.x. [DOI] [PubMed] [Google Scholar]
- McDowall A. W., Hofmann W., Lepault J., Adrian M., Dubochet J. Cryo-electron microscopy of vitrified insect flight muscle. J Mol Biol. 1984 Sep 5;178(1):105–111. doi: 10.1016/0022-2836(84)90233-x. [DOI] [PubMed] [Google Scholar]
- Menetret J. F., Hofmann W., Lepault J. Cryo-electron microscopy of insect flight muscle thick filaments. An approach to dynamic electron microscope studies. J Mol Biol. 1988 Jul 5;202(1):175–178. doi: 10.1016/0022-2836(88)90530-x. [DOI] [PubMed] [Google Scholar]
- Menetret J. F., Schröder R. R., Hofmann W. Cryo-electron microscopic studies of relaxed striated muscle thick filaments. J Muscle Res Cell Motil. 1990 Feb;11(1):1–11. doi: 10.1007/BF01833321. [DOI] [PubMed] [Google Scholar]
- Miller A., Tregear R. T. Structure of insect fibrillar flight muscle in the presence and absence of ATP. J Mol Biol. 1972 Sep 14;70(1):85–104. doi: 10.1016/0022-2836(72)90165-9. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Ménétret J. F., Hofmann W., Schröder R. R., Rapp G., Goody R. S. Time-resolved cryo-electron microscopic study of the dissociation of actomyosin induced by photolysis of photolabile nucleotides. J Mol Biol. 1991 May 20;219(2):139–144. doi: 10.1016/0022-2836(91)90554-j. [DOI] [PubMed] [Google Scholar]
- Reedy M. C., Reedy M. K., Goody R. S. The structure of insect flight muscle in the presence of AMPPNP. J Muscle Res Cell Motil. 1987 Dec;8(6):473–503. doi: 10.1007/BF01567908. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Goody R. S., Hofmann W., Rosenbaum G. Co-ordinated electron microscopy and X-ray studies of glycerinated insect flight muscle. I. X-ray diffraction monitoring during preparation for electron microscopy of muscle fibres fixed in rigor, in ATP and in AMPPNP. J Muscle Res Cell Motil. 1983 Feb;4(1):25–53. doi: 10.1007/BF00711957. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Reedy M. K. Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in the rigor cross-bridge lattice. J Mol Biol. 1968 Jan 28;31(2):155–176. doi: 10.1016/0022-2836(68)90437-3. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., DeRosier D. A surveying optical diffractometer. J Microsc. 1981 Sep;123(Pt 3):239–247. doi: 10.1111/j.1365-2818.1981.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Sjöström M., Squire J. M. Cryo-ultramicrotomy and myofibrillar fine structure: a review. J Microsc. 1977 Dec;111(3):239–278. doi: 10.1111/j.1365-2818.1977.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Sjöström M., Squire J. M. Fine structure of the A-band in cryo-sections. The structure of the A-band of human skeletal muscle fibres from ultra-thin cryo-sections negatively stained. J Mol Biol. 1977 Jan 5;109(1):49–68. doi: 10.1016/s0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]
- Sjöström M., Squire J. M., Luther P., Morris E., Edman A. C. Cryoultramicrotomy of muscle: improved preservation and resolution of muscle ultrastructure using negatively stained ultrathin cryosections. J Microsc. 1991 Jul;163(Pt 1):29–42. doi: 10.1111/j.1365-2818.1991.tb03157.x. [DOI] [PubMed] [Google Scholar]
- TOKUYASU K., OKAMURA S. A new method for making glass knives for thin sectioning. J Biophys Biochem Cytol. 1959 Oct;6:305–308. [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989 Mar;21(3):163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- Trus B. L., Steven A. C., McDowall A. W., Unser M., Dubochet J., Podolsky R. J. Interactions between actin and myosin filaments in skeletal muscle visualized in frozen-hydrated thin sections. Biophys J. 1989 Apr;55(4):713–724. doi: 10.1016/S0006-3495(89)82870-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Yano M. Actomyosin structure in contracting muscle detected by rapid freezing. Nature. 1985 Sep 12;317(6033):182–184. doi: 10.1038/317182a0. [DOI] [PubMed] [Google Scholar]
- Vibert P., Craig R. Electron microscopy and image analysis of myosin filaments from scallop striated muscle. J Mol Biol. 1983 Apr 5;165(2):303–320. doi: 10.1016/s0022-2836(83)80259-9. [DOI] [PubMed] [Google Scholar]
- Vibert P. Helical reconstruction of frozen-hydrated scallop myosin filaments. J Mol Biol. 1992 Feb 5;223(3):661–671. doi: 10.1016/0022-2836(92)90982-p. [DOI] [PubMed] [Google Scholar]
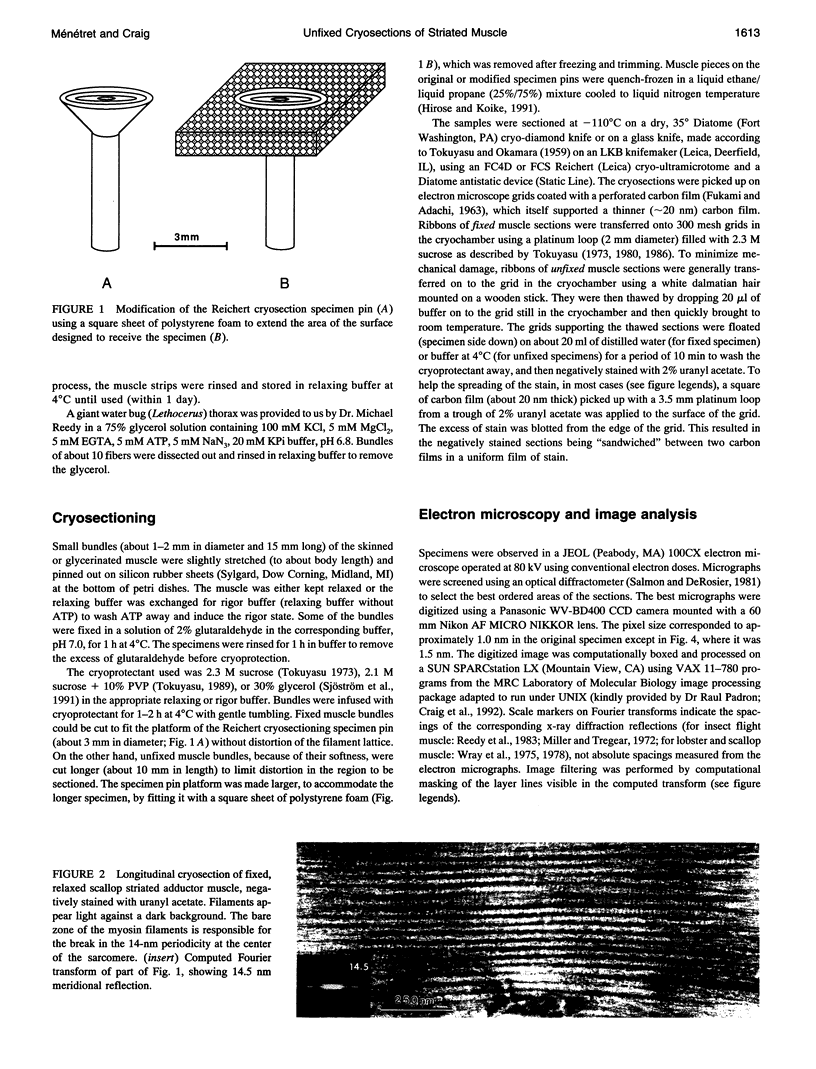
- Wray J. S., Vibert P. J., Cohen C. Diversity of cross-bridge configurations in invertebrate muscles. Nature. 1975 Oct 16;257(5527):561–564. doi: 10.1038/257561a0. [DOI] [PubMed] [Google Scholar]
- Wray J., Vibert P., Cohen C. Actin filaments in muscle: pattern of myosin and tropomyosin/troponin attachments. J Mol Biol. 1978 Sep 25;124(3):501–521. doi: 10.1016/0022-2836(78)90184-5. [DOI] [PubMed] [Google Scholar]