Abstract
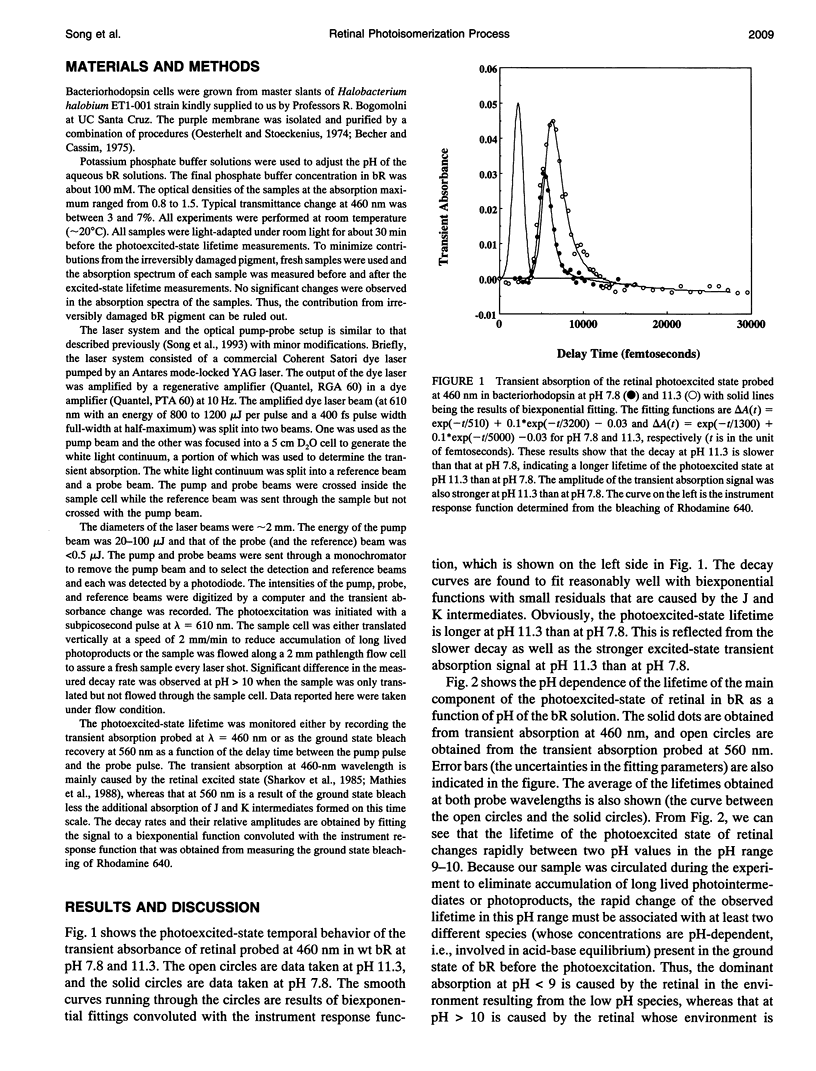
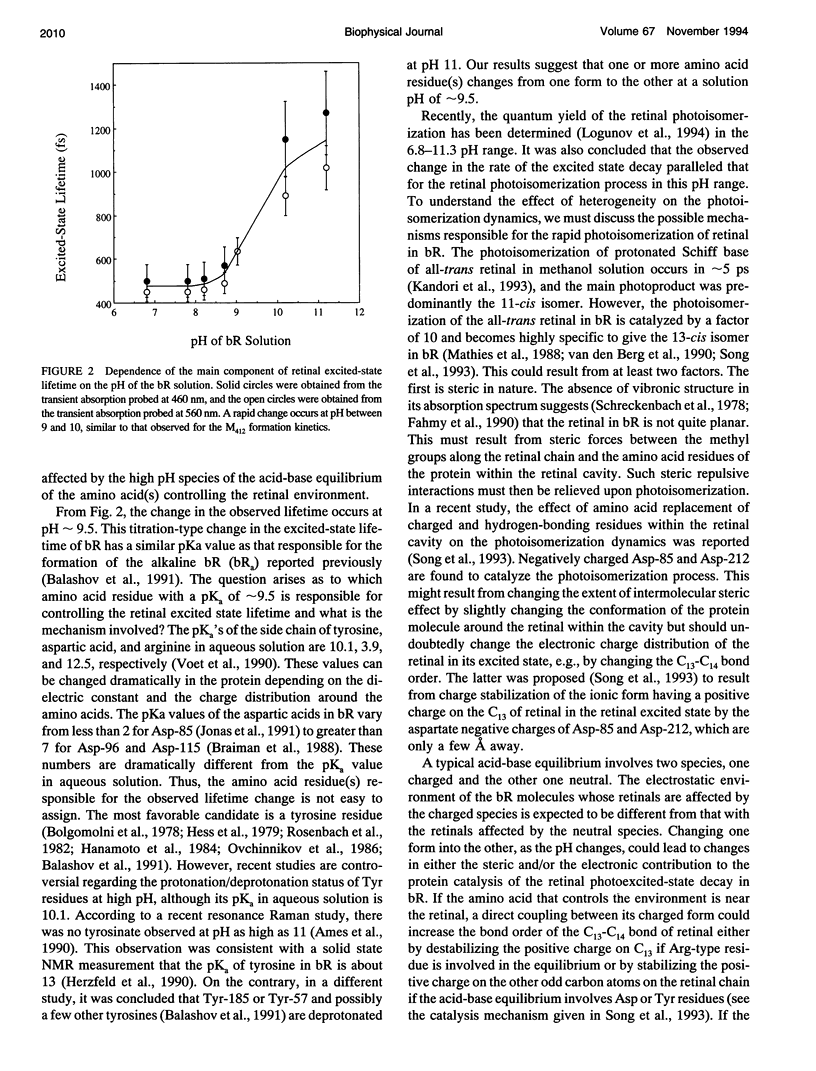
The pH dependence of the subpicosecond decay of the retinal photoexcited state in bacteriorhodopsin (bR) is determined in the pH range 6.8-11.3. A rapid change in the decay rate of the retinal photoexcited state is observed in the pH range 9-10, the same pH range in which a rapid change in the M412 formation kinetics was observed. This observation supports the previously proposed heterogeneity model in which parallel photocycles contribute to the observed pH dependence of the M412 formation kinetics in bR.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Balashov S. P., Govindjee R., Ebrey T. G. Redshift of the purple membrane absorption band and the deprotonation of tyrosine residues at high pH: Origin of the parallel photocycles of trans-bacteriorhodopsin. Biophys J. 1991 Aug;60(2):475–490. doi: 10.1016/S0006-3495(91)82074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Dancsházy Z., Govindjee R., Ebrey T. G. Independent photocycles of the spectrally distinct forms of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6358–6361. doi: 10.1073/pnas.85.17.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Friedman N., Lanyi J. K., Needleman R., Ottolenghi M., Sheves M. The back photoreaction of the M intermediate in the photocycle of bacteriorhodopsin: mechanism and evidence for two M species. Photochem Photobiol. 1992;56(6):1041–1047. doi: 10.1111/j.1751-1097.1992.tb09727.x. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kouyama T. Photoreaction of bacteriorhodopsin at high pH: origins of the slow decay component of M. Biochemistry. 1992 Dec 1;31(47):11740–11747. doi: 10.1021/bi00162a010. [DOI] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld J., Das Gupta S. K., Farrar M. R., Harbison G. S., McDermott A. E., Pelletier S. L., Raleigh D. P., Smith S. O., Winkel C., Lugtenburg J. Solid-state 13C NMR study of tyrosine protonation in dark-adapted bacteriorhodopsin. Biochemistry. 1990 Jun 12;29(23):5567–5574. doi: 10.1021/bi00475a022. [DOI] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. Kinetic interaction between aromatic residues and the retinal chromophore of bacteriorhodopsin during the photocycle. FEBS Lett. 1979 Apr 15;100(2):334–340. doi: 10.1016/0014-5793(79)80364-6. [DOI] [PubMed] [Google Scholar]
- Jonas R., Ebrey T. G. Binding of a single divalent cation directly correlates with the blue-to-purple transition in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):149–153. doi: 10.1073/pnas.88.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komrakov AYu, Kaulen A. D. On the two forms of bacteriorhodopsin. FEBS Lett. 1994 Mar 7;340(3):207–210. doi: 10.1016/0014-5793(94)80139-8. [DOI] [PubMed] [Google Scholar]
- Liu S. Y. Light-induced currents from oriented purple membrane: I. Correlation of the microsecond component (B2) with the L-M photocycle transition. Biophys J. 1990 May;57(5):943–950. doi: 10.1016/S0006-3495(90)82614-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R. A., Brito Cruz C. H., Pollard W. T., Shank C. V. Direct observation of the femtosecond excited-state cis-trans isomerization in bacteriorhodopsin. Science. 1988 May 6;240(4853):777–779. doi: 10.1126/science.3363359. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Braiman M. S., Mogi T., Stern L. J., Khorana H. G. Conserved amino acids in F-helix of bacteriorhodopsin form part of a retinal binding pocket. FEBS Lett. 1989 Jul 3;250(2):448–452. doi: 10.1016/0014-5793(89)80774-4. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Specificity of the retinal binding site of bacteriorhodopsin: chemical and stereochemical requirements for the binding of retinol and retinal. Biochemistry. 1978 Dec 12;17(25):5353–5359. doi: 10.1021/bi00618a005. [DOI] [PubMed] [Google Scholar]
- Song L., El-Sayed M. A., Lanyi J. K. Protein catalysis of the retinal subpicosecond photoisomerization in the primary process of bacteriorhodopsin photosynthesis. Science. 1993 Aug 13;261(5123):891–894. doi: 10.1126/science.261.5123.891. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Pathways of the rise and decay of the M photointermediate(s) of bacteriorhodopsin. Biochemistry. 1990 Mar 6;29(9):2241–2250. doi: 10.1021/bi00461a006. [DOI] [PubMed] [Google Scholar]
- van den Berg R., Du-Jeon-Jang, Bitting H. C., El-Sayed M. A. Subpicosecond resonance Raman spectra of the early intermediates in the photocycle of bacteriorhodopsin. Biophys J. 1990 Jul;58(1):135–141. doi: 10.1016/S0006-3495(90)82359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


