Abstract
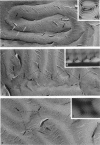
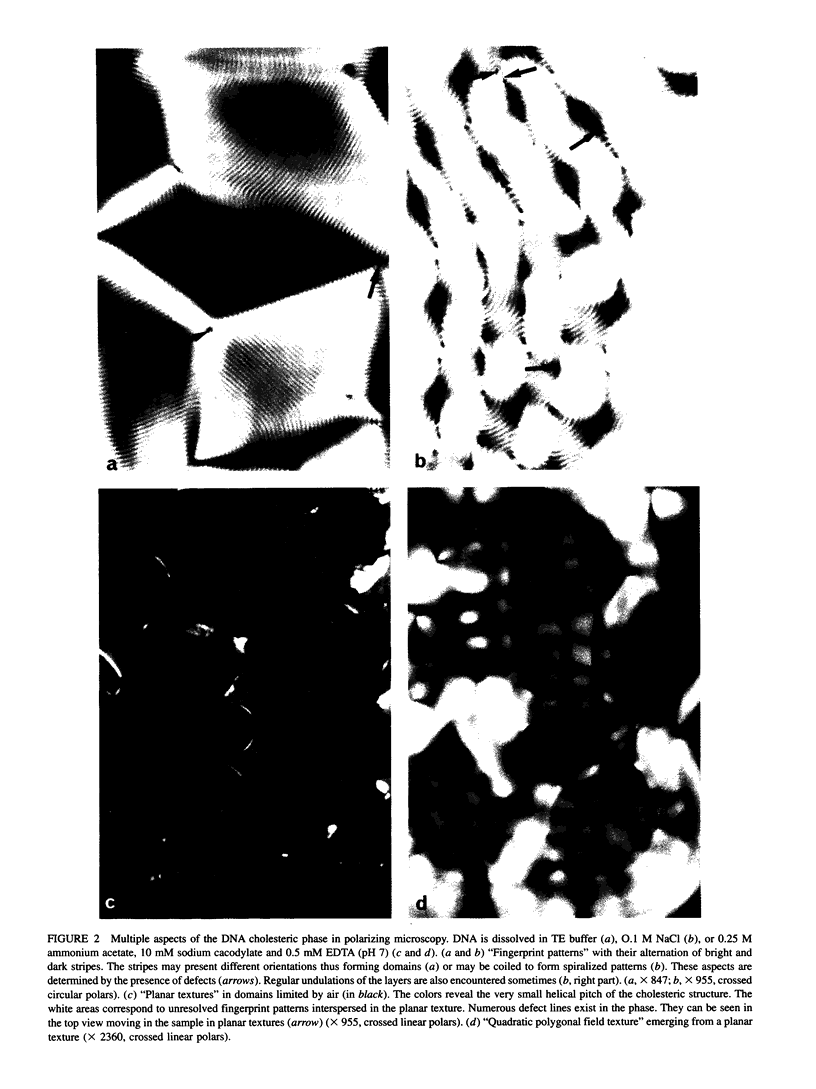
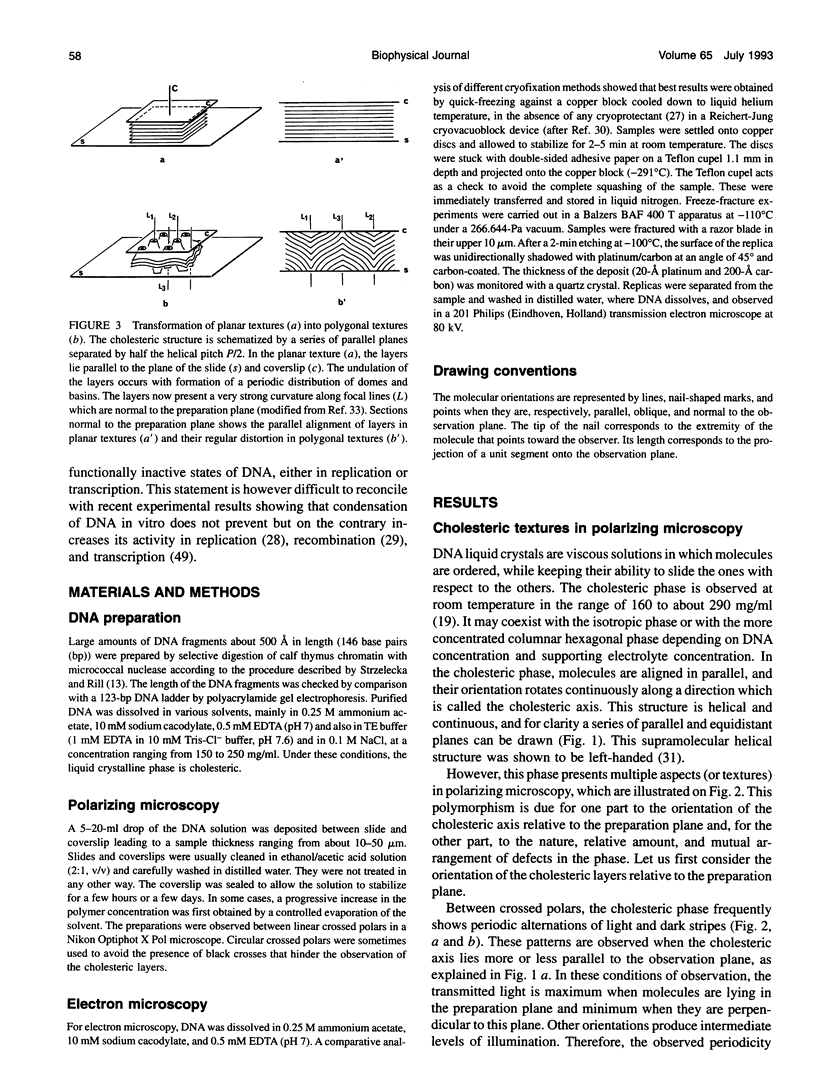
Aqueous solutions of 146-base pair DNA fragments form a cholesteric liquid crystalline phase in the range of about 160-290 mg/ml. We present a structural analysis of this phase by comparing the data obtained from polarizing and electron microscopy. This phase shows multiple aspects or "textures" which are presented and interpreted. They mainly depend on the orientation of the structure relative to the observation plane and on the nature, distribution, and amount of defects present in the phase. These defects are then analyzed with the two methods, and the molecular orientations can be defined precisely in their core. The biological interest of such structural analyses is discussed in relation with the understanding of chromatin structure and function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouligand Y., Soyer M. O., Puiseux-Dao S. La structure fibrillaire et l'orientation des chromosomes chez les Dinoflagellés. Chromosoma. 1968;24(3):251–287. doi: 10.1007/BF00336195. [DOI] [PubMed] [Google Scholar]
- Brandes R., Kearns D. R. Magnetic ordering of DNA liquid crystals. Biochemistry. 1986 Oct 7;25(20):5890–5895. doi: 10.1021/bi00368a008. [DOI] [PubMed] [Google Scholar]
- FEUGHELMAN M., LANGRIDGE R., SEEDS W. E., STOKES A. R., WILSON H. R., HOOPER C. W., WILKINS M. H., BARCLAY R. K., HAMILTON L. D. Molecular structure of deoxyribose nucleic acid and nucleoprotein. Nature. 1955 May 14;175(4463):834–838. [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Catenation of DNA rings by topoisomerases. Mechanism of control by spermidine. J Biol Chem. 1982 Mar 10;257(5):2687–2693. [PubMed] [Google Scholar]
- LUZZATI V., NICOLAUIEFF A. THE STRUCTURE OF NUCLEOHISTONES AND NUCLEOPROTAMINES. J Mol Biol. 1963 Aug;7:142–163. doi: 10.1016/s0022-2836(63)80043-1. [DOI] [PubMed] [Google Scholar]
- Leforestier A., Livolant F. Cholesteric liquid crystalline DNA; a comparative analysis of cryofixation methods. Biol Cell. 1991;71(1-2):115–122. doi: 10.1016/0248-4900(91)90058-u. [DOI] [PubMed] [Google Scholar]
- Lepault J., Dubochet J., Baschong W., Kellenberger E. Organization of double-stranded DNA in bacteriophages: a study by cryo-electron microscopy of vitrified samples. EMBO J. 1987 May;6(5):1507–1512. doi: 10.1002/j.1460-2075.1987.tb02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman L. S. Chromosomal analogues: long-range order in psi-condensed DNA. Cold Spring Harb Symp Quant Biol. 1974;38:59–73. doi: 10.1101/sqb.1974.038.01.009. [DOI] [PubMed] [Google Scholar]
- Livolant F. Cholesteric organization of DNA in vivo and in vitro. Eur J Cell Biol. 1984 Mar;33(2):300–311. [PubMed] [Google Scholar]
- Livolant F., Levelut A. M., Doucet J., Benoit J. P. The highly concentrated liquid-crystalline phase of DNA is columnar hexagonal. Nature. 1989 Jun 29;339(6227):724–726. doi: 10.1038/339724a0. [DOI] [PubMed] [Google Scholar]
- Livolant F., Maestre M. F. Circular dichroism microscopy of compact forms of DNA and chromatin in vivo and in vitro: cholesteric liquid-crystalline phases of DNA and single dinoflagellate nuclei. Biochemistry. 1988 Apr 19;27(8):3056–3068. doi: 10.1021/bi00408a058. [DOI] [PubMed] [Google Scholar]
- Livolant F. Supramolecular organization of double-stranded DNA molecules in the columnar hexagonal liquid crystalline phase. An electron microscopic analysis using freeze-fracture methods. J Mol Biol. 1991 Mar 5;218(1):165–181. doi: 10.1016/0022-2836(91)90882-7. [DOI] [PubMed] [Google Scholar]
- Pheiffer B. H., Zimmerman S. B. Polymer-stimulated ligation: enhanced blunt- or cohesive-end ligation of DNA or deoxyribooligonucleotides by T4 DNA ligase in polymer solutions. Nucleic Acids Res. 1983 Nov 25;11(22):7853–7871. doi: 10.1093/nar/11.22.7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rill R. L. Liquid crystalline phases in concentrated aqueous solutions of Na+ DNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):342–346. doi: 10.1073/pnas.83.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rill R. L., Livolant F., Aldrich H. C., Davidson M. W. Electron microscopy of liquid crystalline DNA: direct evidence for cholesteric-like organization of DNA in dinoflagellate chromosomes. Chromosoma. 1989 Oct;98(4):280–286. doi: 10.1007/BF00327314. [DOI] [PubMed] [Google Scholar]
- Sikorav J. L., Church G. M. Complementary recognition in condensed DNA: accelerated DNA renaturation. J Mol Biol. 1991 Dec 20;222(4):1085–1108. doi: 10.1016/0022-2836(91)90595-w. [DOI] [PubMed] [Google Scholar]
- Strzelecka T. E., Davidson M. W., Rill R. L. Multiple liquid crystal phases of DNA at high concentrations. Nature. 1988 Feb 4;331(6155):457–460. doi: 10.1038/331457a0. [DOI] [PubMed] [Google Scholar]
- Strzelecka T. E., Rill R. L. Phase transitions of concentrated DNA solutions in low concentrations of 1:1 supporting electrolyte. Biopolymers. 1990;30(1-2):57–71. doi: 10.1002/bip.360300108. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Replication of lambda dv plasmid in vitro promoted by purified lambda O and P proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7639–7643. doi: 10.1073/pnas.79.24.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Harrison B. Macromolecular crowding increases binding of DNA polymerase to DNA: an adaptive effect. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1871–1875. doi: 10.1073/pnas.84.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]