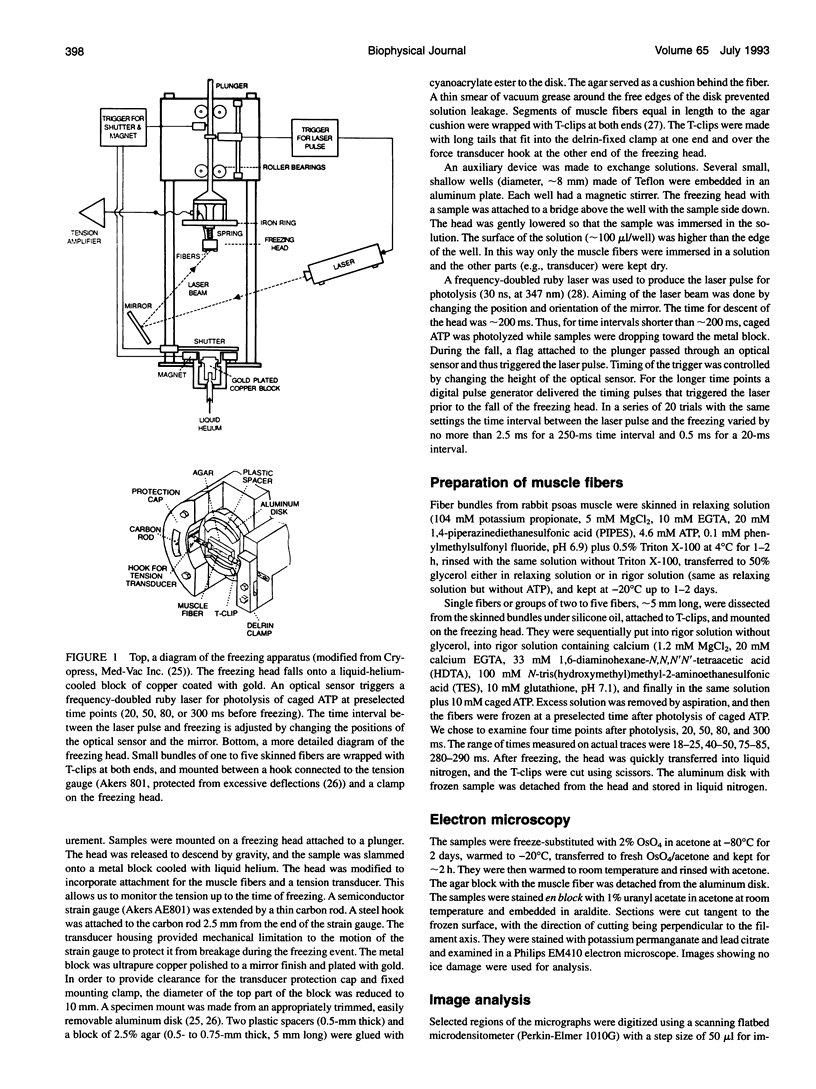
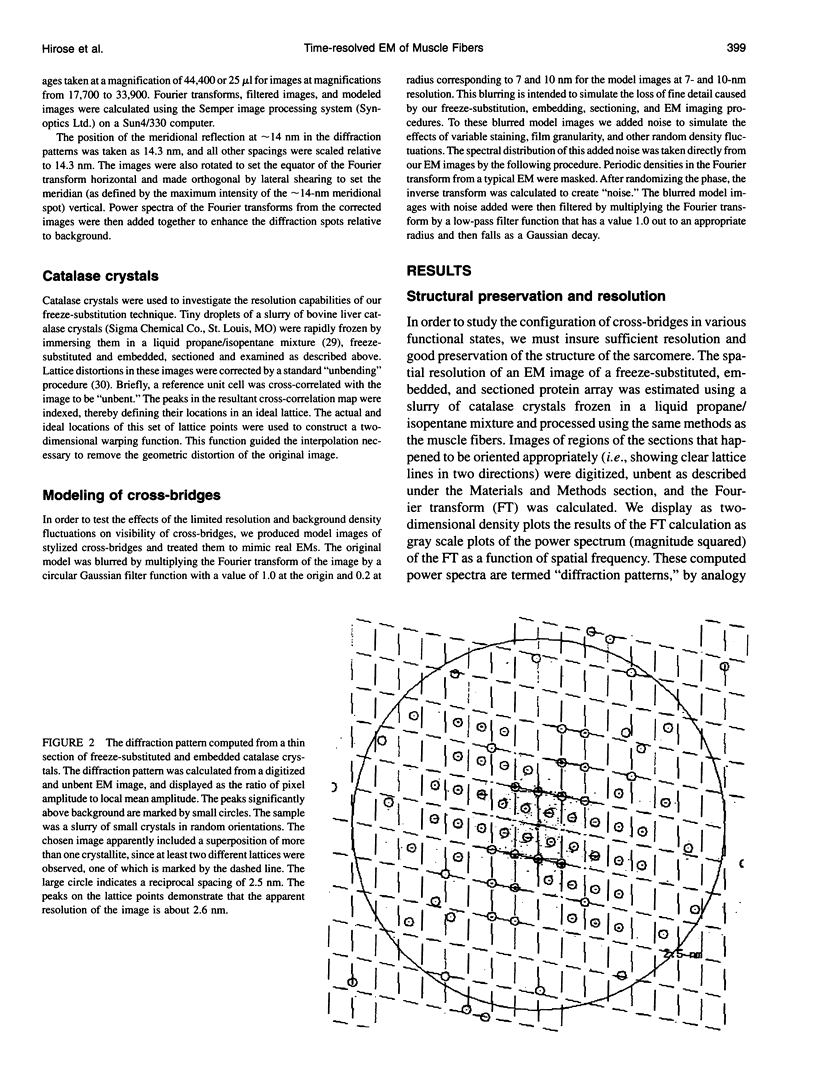
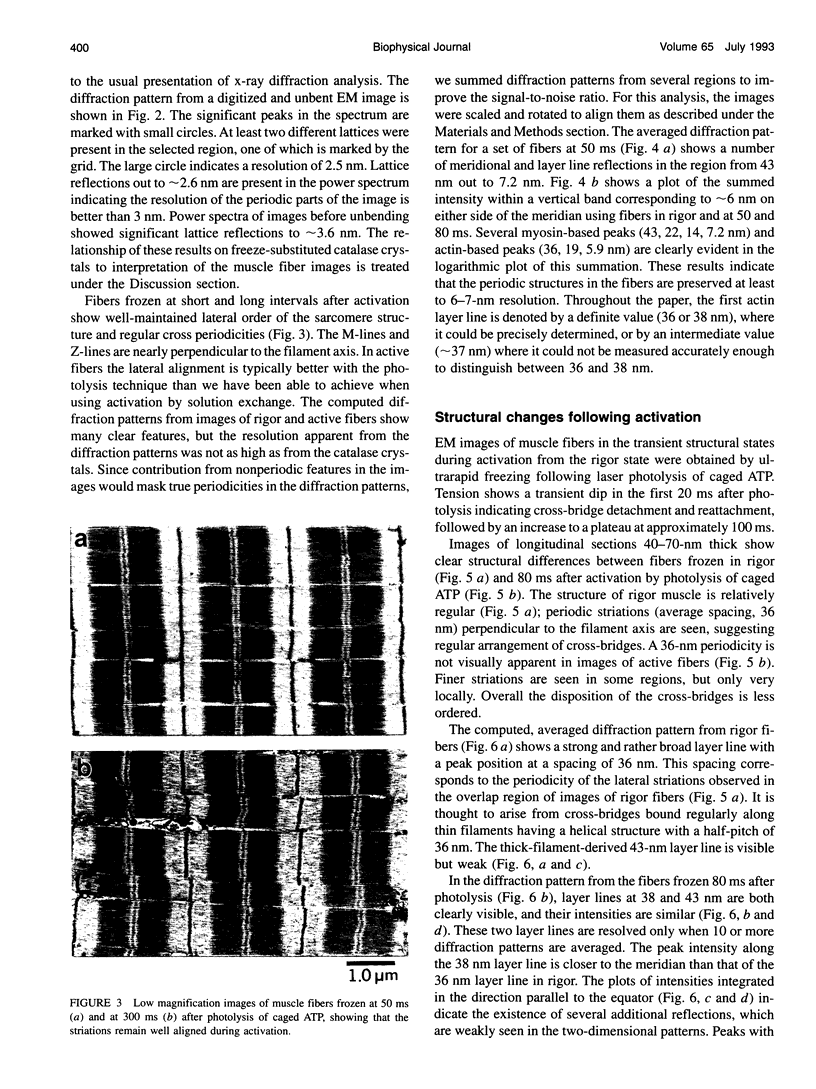
Abstract
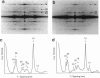
A new approach was used to study transient structural states of cross-bridges during activation of muscle fibers. Rabbit skinned muscle fibers were rapidly and synchronously activated from the rigor state by photolysis of caged ATP in the presence of Ca2+. At several different times during the switch from rigor to fully active tension development, the fibers were rapidly frozen on a liquid helium-cooled metal block, freeze-substituted, and examined in an electron microscope. The limits of structural preservation and resolution with this technique were analyzed. We demonstrate that the resolution of our images is sufficient to draw the following conclusions about cross-bridge structure. Rigor cross-bridges point away from the Z-line and most of them are wider near the thin filaments than near the backbone of the thick filaments. In contrast, cross-bridges in actively contracting fibers stretch between the thick and thin filaments at a variable angle, and are uniformly thin. Diffraction patterns computed from contracting muscle show layer lines both at 38 and 43 nm indicating that active cross-bridges contribute mass to both the actin- and myosin-based helical periodicities. The images obtained from fibers frozen 20 ms after release of ATP show a mixture of rigor and active type cross-bridge configurations. There is little evidence of cross-bridges with the rigor shape by 50 ms, and the difference in configurations between 50 and 300 ms after photolysis is surprisingly subtle.
Full text
PDF
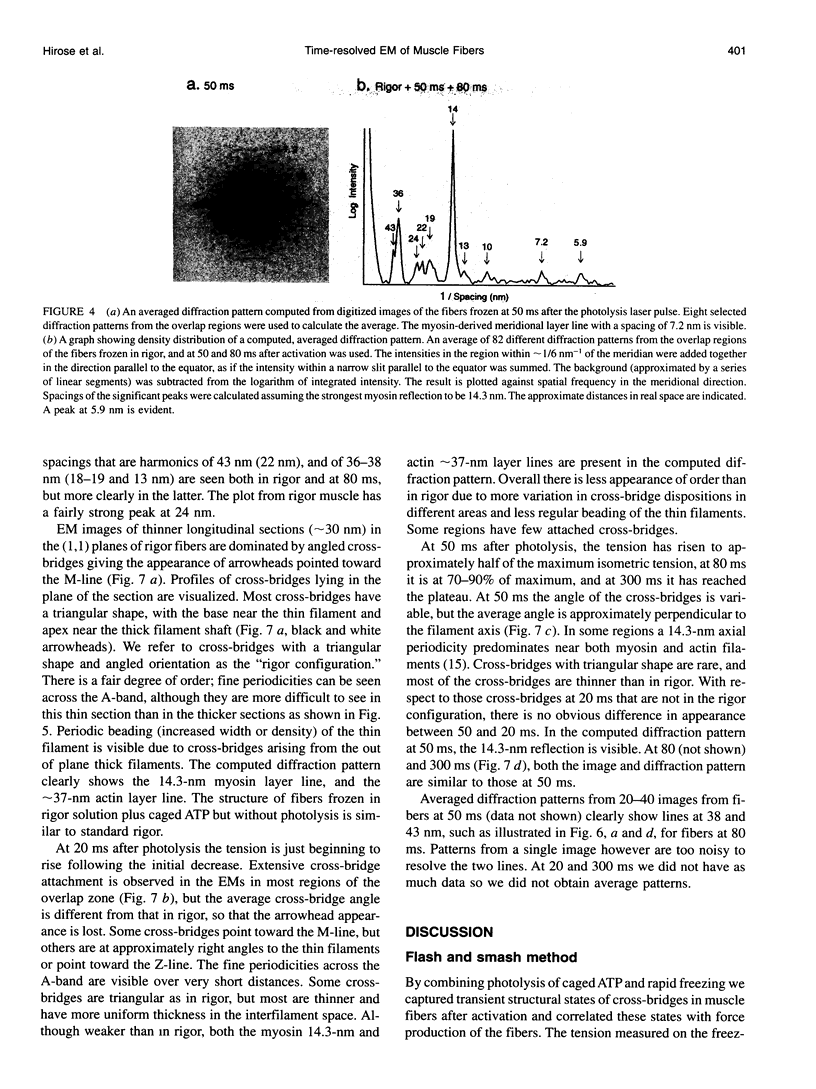
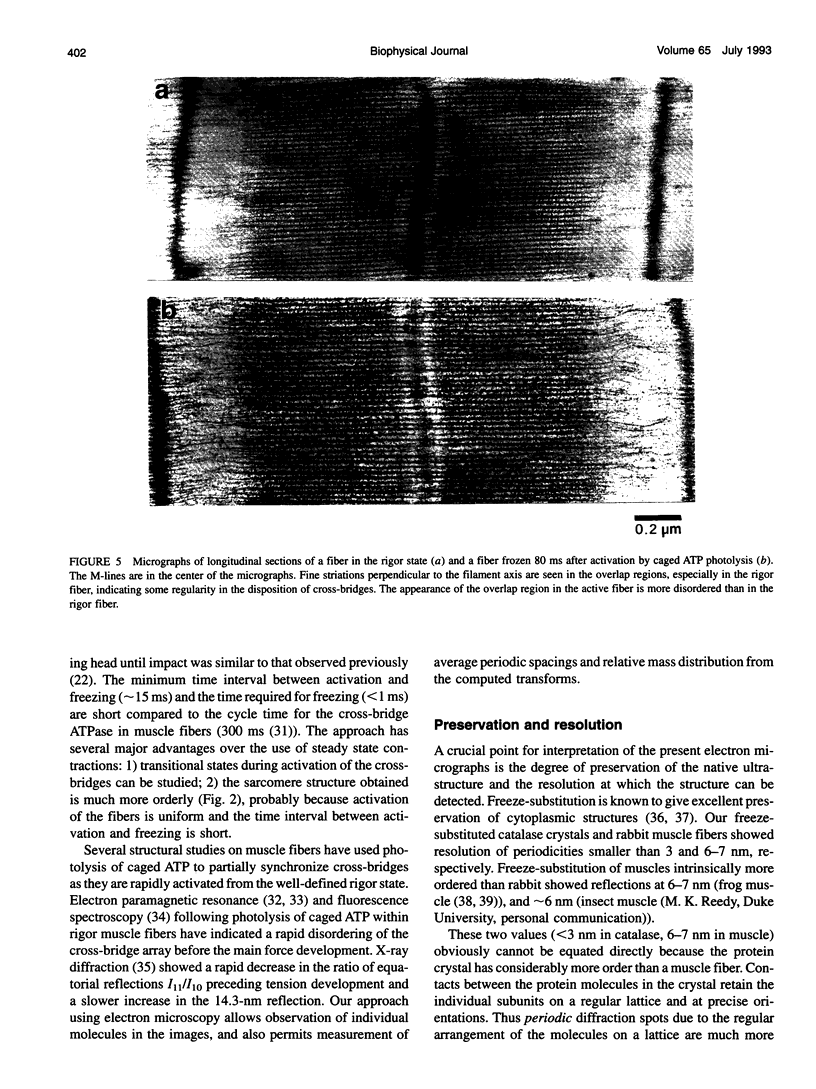
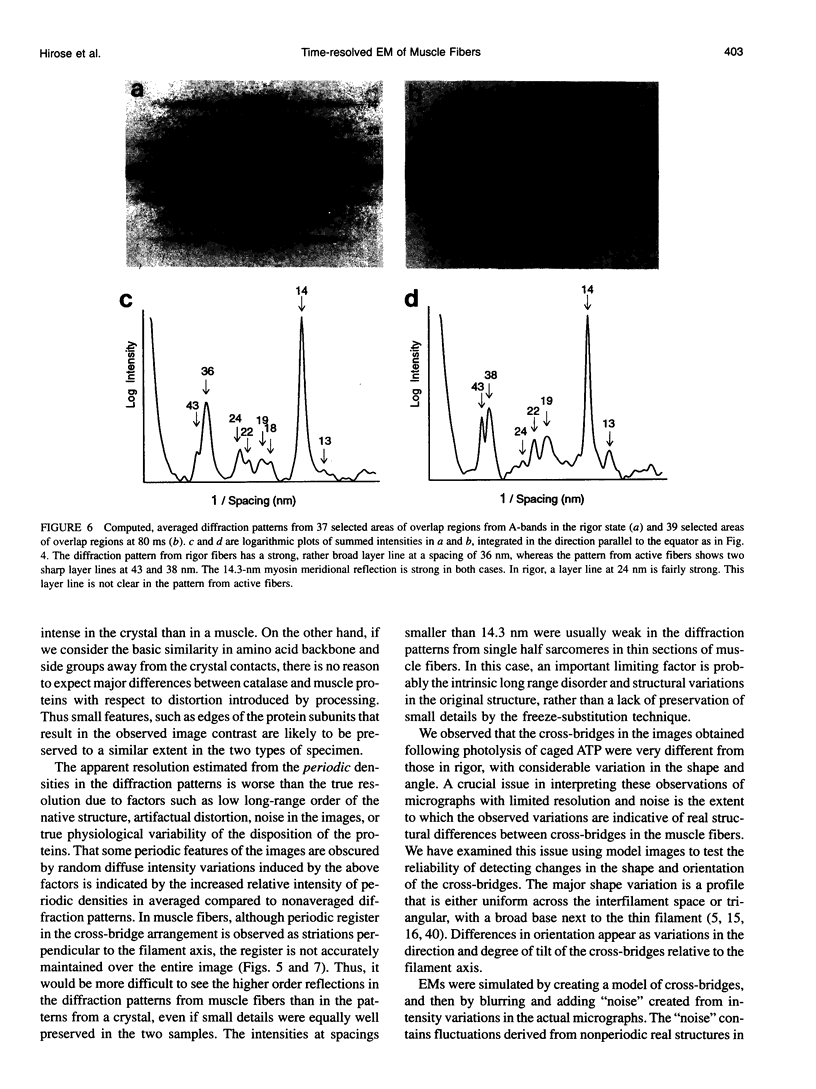
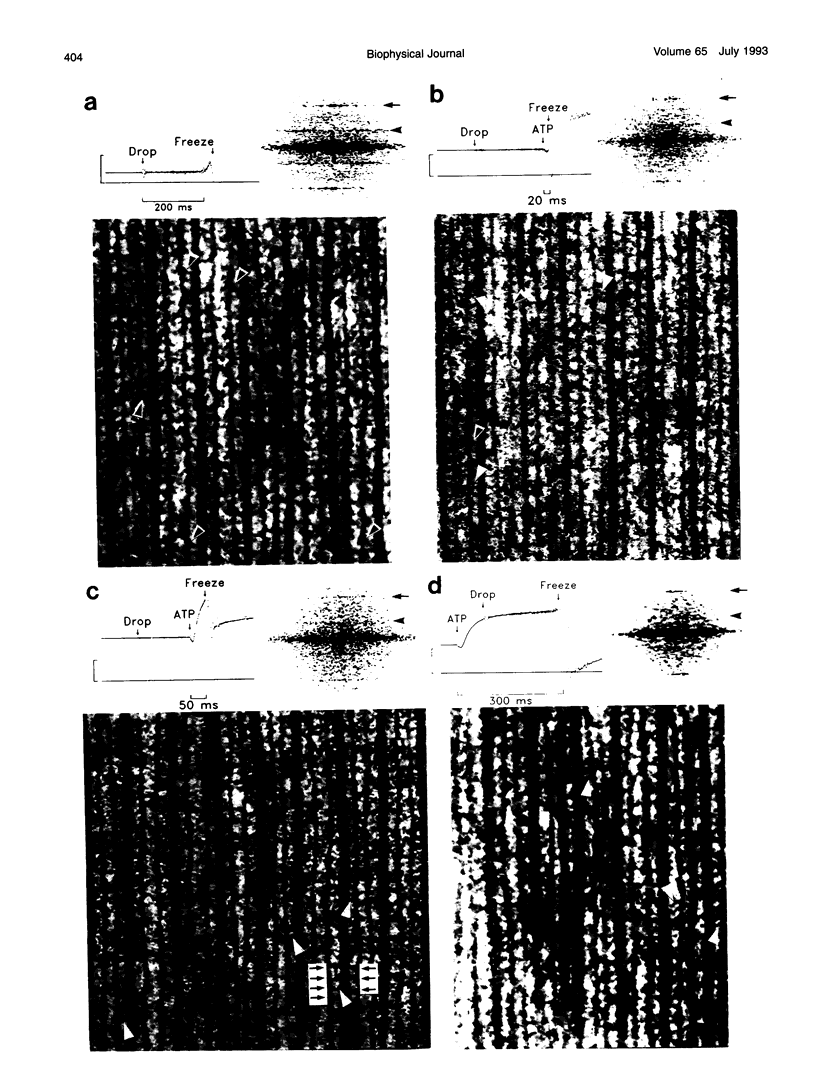




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applegate D., Flicker P. New states of actomyosin. J Biol Chem. 1987 May 15;262(14):6856–6863. [PubMed] [Google Scholar]
- Bard F., Franzini-Armstrong C., Ip W. Rigor crossbridges are double-headed in fast muscle from crayfish. J Cell Biol. 1987 Nov;105(5):2225–2234. doi: 10.1083/jcb.105.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Svensson E. C., Thomas D. D. Photolysis of a photolabile precursor of ATP (caged ATP) induces microsecond rotational motions of myosin heads bound to actin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8753–8757. doi: 10.1073/pnas.86.22.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Thomas D. D. Rotational dynamics of actin-bound myosin heads in active myofibrils. Biochemistry. 1993 Apr 13;32(14):3812–3821. doi: 10.1021/bi00065a038. [DOI] [PubMed] [Google Scholar]
- Bordas J., Diakun G. P., Harries J. E., Lewis R. A., Mant G. R., Martin-Fernandez M. L., Towns-Andrews E. Two-dimensional time resolved X-ray diffraction of muscle: recent results. Adv Biophys. 1991;27:15–33. doi: 10.1016/0065-227x(91)90005-x. [DOI] [PubMed] [Google Scholar]
- Bridgman P. C., Reese T. S. The structure of cytoplasm in directly frozen cultured cells. I. Filamentous meshworks and the cytoplasmic ground substance. J Cell Biol. 1984 Nov;99(5):1655–1668. doi: 10.1083/jcb.99.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Craig R., Alamo L., Padrón R. Structure of the myosin filaments of relaxed and rigor vertebrate striated muscle studied by rapid freezing electron microscopy. J Mol Biol. 1992 Nov 20;228(2):474–487. doi: 10.1016/0022-2836(92)90836-9. [DOI] [PubMed] [Google Scholar]
- Craig R., Greene L. E., Eisenberg E. Structure of the actin-myosin complex in the presence of ATP. Proc Natl Acad Sci U S A. 1985 May;82(10):3247–3251. doi: 10.1073/pnas.82.10.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Brunsvold N. J., Thomas D. D. Effects of AMPPNP on the orientation and rotational dynamics of spin-labeled muscle cross-bridges. Biophys J. 1988 Apr;53(4):513–524. doi: 10.1016/S0006-3495(88)83131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Thomas D. D. Myosin heads have a broad orientational distribution during isometric muscle contraction: time-resolved EPR studies using caged ATP. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5538–5542. doi: 10.1073/pnas.87.14.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Trentham D. R. The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J Physiol. 1984 Jul;352:575–599. doi: 10.1113/jphysiol.1984.sp015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during the rise of tetanic tension in frog muscle fibres. J Physiol. 1986 Mar;372:595–609. doi: 10.1113/jphysiol.1986.sp016027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frado L. L., Craig R. Electron microscopy of the actin-myosin head complex in the presence of ATP. J Mol Biol. 1992 Jan 20;223(2):391–397. doi: 10.1016/0022-2836(92)90659-8. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Initiation of active contraction by photogeneration of adenosine-5'-triphosphate in rabbit psoas muscle fibres. J Physiol. 1984 Sep;354:605–624. doi: 10.1113/jphysiol.1984.sp015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E. Kinetics of the actomyosin ATPase in muscle fibers. Annu Rev Physiol. 1987;49:637–654. doi: 10.1146/annurev.ph.49.030187.003225. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J Physiol. 1984 May;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990 Nov 5;216(1):49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Reedy M. K. Modeling rigor cross-bridge patterns in muscle I. Initial studies of the rigor lattice of insect flight muscle. Biophys J. 1978 Dec;24(3):713–728. doi: 10.1016/S0006-3495(78)85415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E. Structure of the myosin crossbridge lattice in insect flight muscle. J Mol Biol. 1983 Sep 5;169(1):123–154. doi: 10.1016/s0022-2836(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Hirose K., Wakabayashi T. Conformations of crossbridges in contracting skeletal muscle. Adv Biophys. 1991;27:197–203. doi: 10.1016/0065-227x(91)90018-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Irving M., Lombardi V., Piazzesi G., Ferenczi M. A. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature. 1992 May 14;357(6374):156–158. doi: 10.1038/357156a0. [DOI] [PubMed] [Google Scholar]
- Katayama E. The effects of various nucleotides on the structure of actin-attached myosin subfragment-1 studied by quick-freeze deep-etch electron microscopy. J Biochem. 1989 Nov;106(5):751–770. doi: 10.1093/oxfordjournals.jbchem.a122928. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Lepault J., Erk I., Nicolas G., Ranck J. L. Time-resolved cryo-electron microscopy of vitrified muscular components. J Microsc. 1991 Jan;161(Pt 1):47–57. doi: 10.1111/j.1365-2818.1991.tb03072.x. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Flicker P. F. Structural relationships of actin, myosin, and tropomyosin revealed by cryo-electron microscopy. J Cell Biol. 1987 Jul;105(1):29–39. doi: 10.1083/jcb.105.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Huxley H. E., DeRosier D. J. Three-dimensional reconstruction of F-actin, thin filaments and decorated thin filaments. J Mol Biol. 1970 Jun 14;50(2):279–295. doi: 10.1016/0022-2836(70)90192-0. [DOI] [PubMed] [Google Scholar]
- Padrón R., Alamo L., Craig R., Caputo C. A method for quick-freezing live muscles at known instants during contraction with simultaneous recording of mechanical tension. J Microsc. 1988 Aug;151(Pt 2):81–102. doi: 10.1111/j.1365-2818.1988.tb04616.x. [DOI] [PubMed] [Google Scholar]
- Padrón R., Huxley H. E. The effect of the ATP analogue AMPPNP on the structure of crossbridges in vertebrate skeletal muscles: X-ray diffraction and mechanical studies. J Muscle Res Cell Motil. 1984 Dec;5(6):613–655. doi: 10.1007/BF00713923. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Bhandari D., Maupin P., Wachsstock D., Weeds A. G., Zot H. G. Direct visualization by electron microscopy of the weakly bound intermediates in the actomyosin adenosine triphosphatase cycle. Biophys J. 1993 Feb;64(2):454–471. doi: 10.1016/S0006-3495(93)81387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K. J., Maeda Y., Rapp G., Goody R. S. Dynamic X-ray diffraction measurements following photolytic relaxation and activation of skinned rabbit psoas fibres. Adv Biophys. 1991;27:63–75. doi: 10.1016/0065-227x(91)90008-2. [DOI] [PubMed] [Google Scholar]
- Reedy M. C., Reedy M. K., Goody R. S. The structure of insect flight muscle in the presence of AMPPNP. J Muscle Res Cell Motil. 1987 Dec;8(6):473–503. doi: 10.1007/BF01567908. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Reedy M. C. Rigor crossbridge structure in tilted single filament layers and flared-X formations from insect flight muscle. J Mol Biol. 1985 Sep 5;185(1):145–176. doi: 10.1016/0022-2836(85)90188-3. [DOI] [PubMed] [Google Scholar]
- Reedy M. K. Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in the rigor cross-bridge lattice. J Mol Biol. 1968 Jan 28;31(2):155–176. doi: 10.1016/0022-2836(68)90437-3. [DOI] [PubMed] [Google Scholar]
- Stein R. A., Ludescher R. D., Dahlberg P. S., Fajer P. G., Bennett R. L., Thomas D. D. Time-resolved rotational dynamics of phosphorescent-labeled myosin heads in contracting muscle fibers. Biochemistry. 1990 Oct 30;29(43):10023–10031. doi: 10.1021/bi00495a003. [DOI] [PubMed] [Google Scholar]
- Tanner J. W., Thomas D. D., Goldman Y. E. Transients in orientation of a fluorescent cross-bridge probe following photolysis of caged nucleotides in skeletal muscle fibres. J Mol Biol. 1992 Jan 5;223(1):185–203. doi: 10.1016/0022-2836(92)90725-y. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Amos L. A. A new model for the geometry of the binding of myosin crossbridges to muscle thin filaments. J Mol Biol. 1981 Apr 5;147(2):297–324. doi: 10.1016/0022-2836(81)90442-3. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Reedy M. C., Córdova L., Reedy M. K. Three-dimensional image reconstruction of insect flight muscle. I. The rigor myac layer. J Cell Biol. 1989 Sep;109(3):1085–1102. doi: 10.1083/jcb.109.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima C., Wakabayashi T. Three-dimensional image analysis of the complex of thin filaments and myosin molecules from skeletal muscle. IV. Reconstitution from minimal- and high-dose images of the actin-tropomyosin-myosin subfragment-1 complex. J Biochem. 1985 Jan;97(1):219–243. doi: 10.1093/oxfordjournals.jbchem.a135048. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Yano M. Actomyosin structure in contracting muscle detected by rapid freezing. Nature. 1985 Sep 12;317(6033):182–184. doi: 10.1038/317182a0. [DOI] [PubMed] [Google Scholar]
- Varriano-Marston E., Franzini-Armstrong C., Haselgrove J. C. The structure and disposition of crossbridges in deep-etched fish muscle. J Muscle Res Cell Motil. 1984 Aug;5(4):363–386. doi: 10.1007/BF00818256. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Tanaka H., Saito H., Moriwaki N., Ueno Y., Amemiya Y. Dynamic X-ray diffraction of skeletal muscle contraction: structural change of actin filaments. Adv Biophys. 1991;27:3–13. doi: 10.1016/0065-227x(91)90004-w. [DOI] [PubMed] [Google Scholar]
- Yagi N. Intensification of the first actin layer-line during contraction of frog skeletal muscle. Adv Biophys. 1991;27:35–43. doi: 10.1016/0065-227x(91)90006-y. [DOI] [PubMed] [Google Scholar]