Abstract
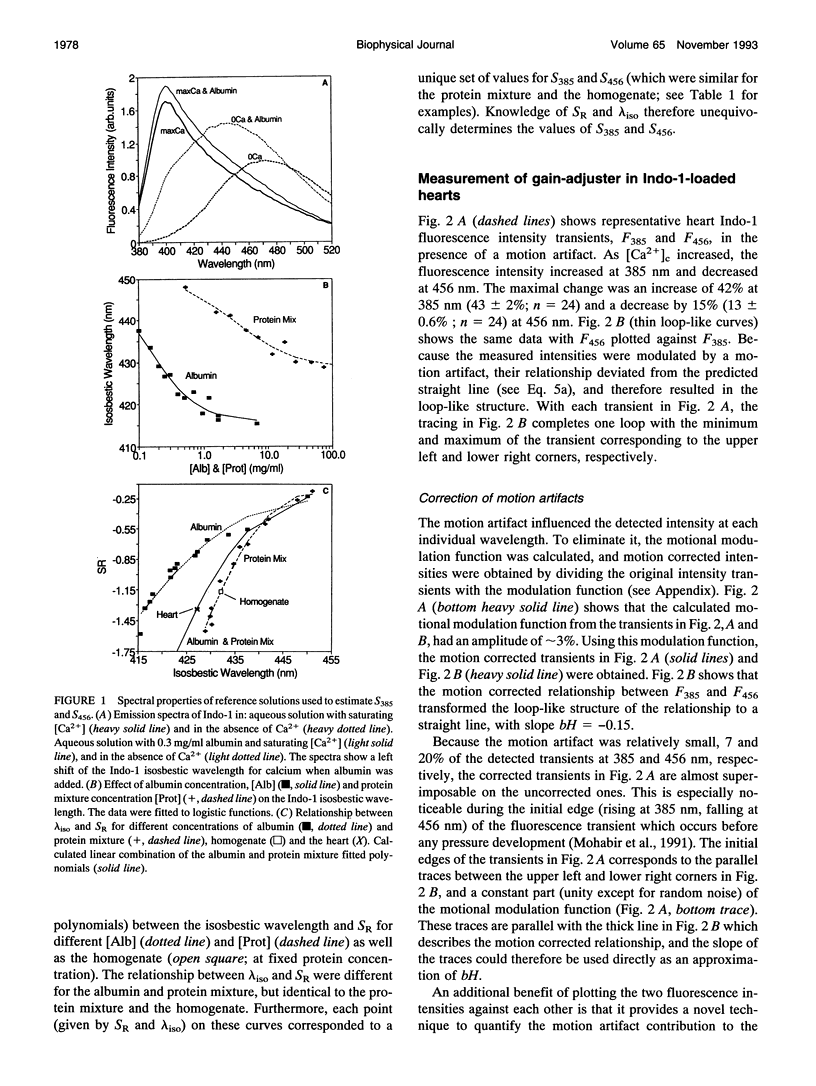
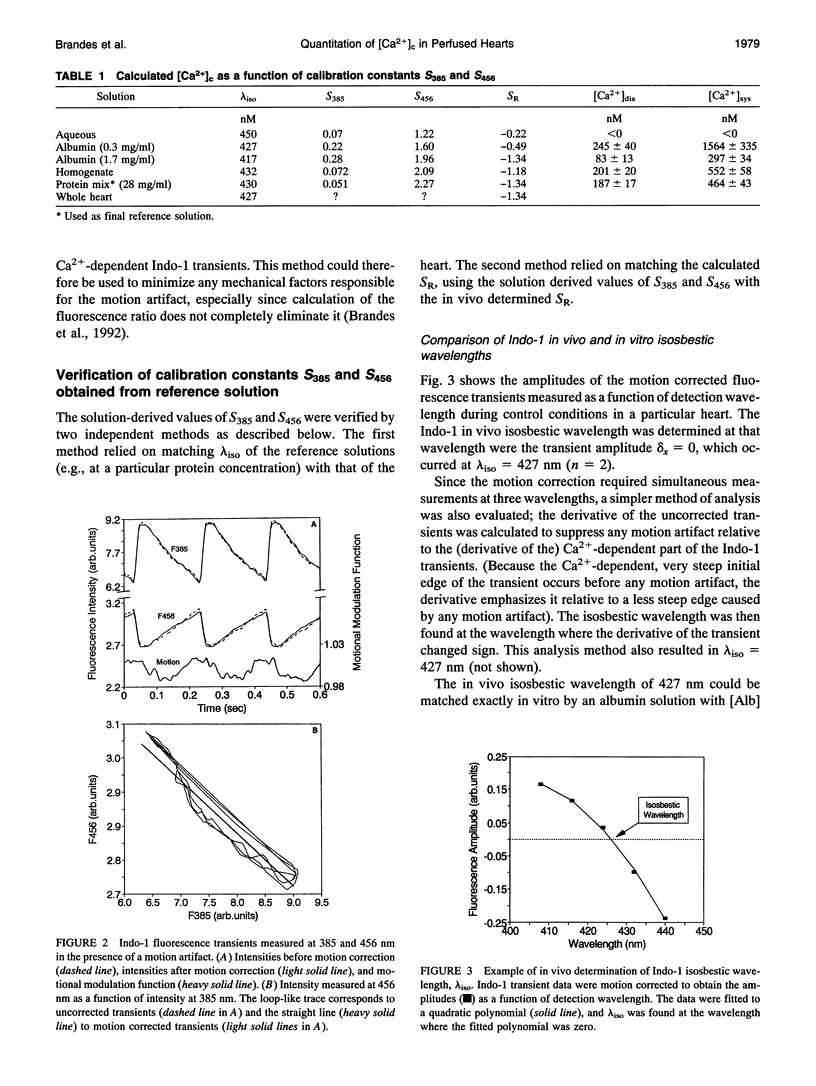
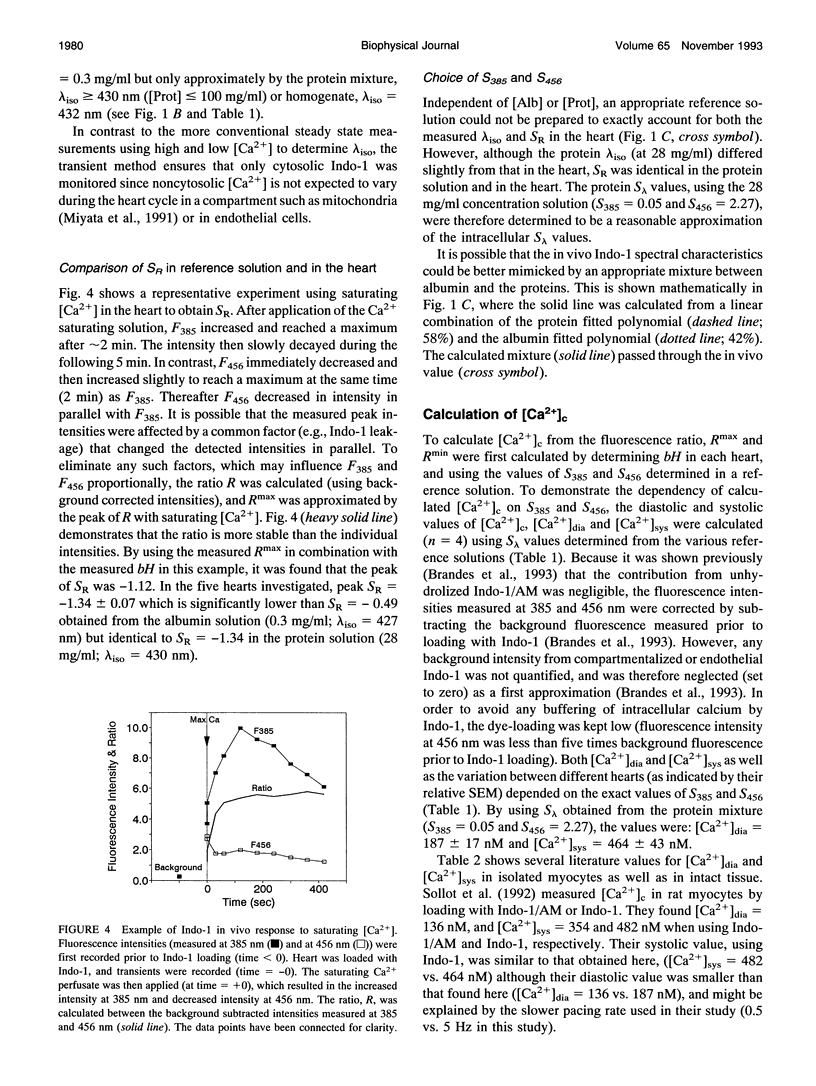
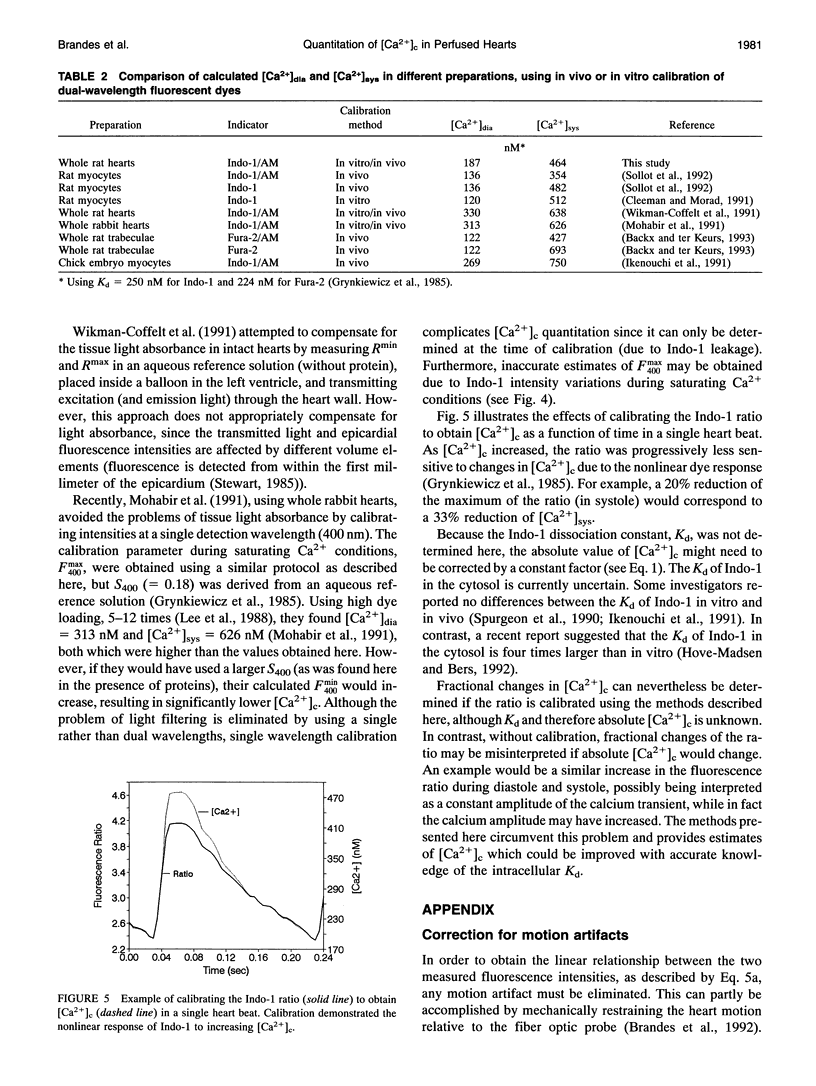
Fluorometric determination of cytosolic calcium, [Ca2+]c, using Indo-1 in intact tissue, is limited by problems in obtaining calibration parameters for Indo-1 in vivo. Therefore, the goal of this study was to calibrate Indo-1 using in vitro constants, obtained from protein-containing reference solutions designed to produce similar Indo-1 spectral properties to those in vivo. Due to wavelength-dependent tissue light absorbance, the in vitro constants had to be absorbance-corrected using a novel method. The correction factor was calculated from the relationship between the Indo-1 fluorescence intensities at the two detection wavelengths. A mixture of proteins at approximately 28 mg/ml had a similar Indo-1 isosbestic wavelength (430 nm) to that found in vivo (427 nm), and a similar fluorescence ratio maximum with saturating Ca2+ to that found in vivo (after absorbance correction). Using calibration constants from this protein mixture, calculated [Ca2+]c in a Langendorf perfused rat heart was 187 nM during diastole, and 464 nM in systole. This new calibration method circumvented the considerable experimental problems of previous methods which required measurements with the cytosol fully depleted and fully saturated with Ca2+.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleemann L., Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. J Physiol. 1991 Jan;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fralix T. A., Heineman F. W., Balaban R. S. Effects of tissue absorbance on NAD(P)H and Indo-1 fluorescence from perfused rabbit hearts. FEBS Lett. 1990 Mar 26;262(2):287–292. doi: 10.1016/0014-5793(90)80212-2. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hove-Madsen L., Bers D. M. Indo-1 binding to protein in permeabilized ventricular myocytes alters its spectral and Ca binding properties. Biophys J. 1992 Jul;63(1):89–97. doi: 10.1016/S0006-3495(92)81597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi H., Peeters G. A., Barry W. H. Evidence that binding of Indo-1 to cardiac myocyte protein does not markedly change Kd for Ca2+. Cell Calcium. 1991 Jun;12(6):415–422. doi: 10.1016/0143-4160(91)90067-o. [DOI] [PubMed] [Google Scholar]
- Kihara Y., Grossman W., Morgan J. P. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res. 1989 Oct;65(4):1029–1044. doi: 10.1161/01.res.65.4.1029. [DOI] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Koretsune Y., Yue D. T., Chacko V. P., Pike M. M. Quantification of [Ca2+]i in perfused hearts. Critical evaluation of the 5F-BAPTA and nuclear magnetic resonance method as applied to the study of ischemia and reperfusion. Circ Res. 1990 May;66(5):1255–1267. doi: 10.1161/01.res.66.5.1255. [DOI] [PubMed] [Google Scholar]
- Mohabir R., Lee H. C., Kurz R. W., Clusin W. T. Effects of ischemia and hypercarbic acidosis on myocyte calcium transients, contraction, and pHi in perfused rabbit hearts. Circ Res. 1991 Dec;69(6):1525–1537. doi: 10.1161/01.res.69.6.1525. [DOI] [PubMed] [Google Scholar]
- Owen C. S., Shuler R. L. Spectral evidence for non-calcium interactions of intracellular Indo-1. Biochem Biophys Res Commun. 1989 Aug 30;163(1):328–333. doi: 10.1016/0006-291x(89)92139-6. [DOI] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990 Feb-Mar;11(2-3):85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- Stewart A. W. Muscle fluorometry: a determination of the depth of penetration. Experientia. 1985 Apr 15;41(4):456–458. doi: 10.1007/BF01966144. [DOI] [PubMed] [Google Scholar]
- Wikman-Coffelt J., Wu S. T., Parmley W. W. Intracellular endocardial calcium and myocardial function in rat hearts. Cell Calcium. 1991 Jan;12(1):39–50. doi: 10.1016/0143-4160(91)90083-q. [DOI] [PubMed] [Google Scholar]


