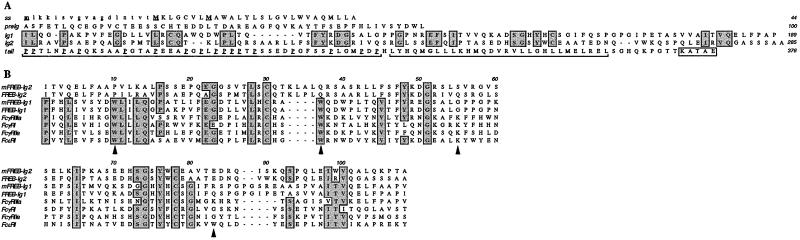
Figure 1.
Predicted amino acid sequences of FREB. (A) FREB consists of a putative signal peptide (ss) followed by a hydrophilic peptide (pre-Ig) that precedes two Ig-SF domains (Ig1 and Ig2). FREB ends with a tail consisting of a proline-rich region, a leucine-rich region (both highlighted by brackets), and a putative endoplasmic reticulum retention signal (boxed). Potential starting methionines are marked in bold and underlined. The putative signal sequence after the second and third starting methionines is indicated in capital letters. Conserved residues between the two Ig-SF folds are boxed, including the cysteines potentially involved in generating the intrachain disulfide bridge of the Ig-SF fold. Proline residues in the tail are underlined. FREB lacks any N-linked glycosylation site. Human and mouse FREB sequences have been submitted to GenBank database under accession nos. AF426461 and AF426462, respectively. (B) Alignment of human and mouse FREB Ig-SF domains with the membrane proximal Ig-SF domains of human Fc receptors. Arrows indicate the four Fc receptor interface residues that are invariant among all human FcγRs. The pre-Ig domain has a limited sequence identity to a classical Ig-fold (data not shown), although the fold topology could not be adopted because of the deletion of several core elements.