Abstract
Heat shock proteins (Hsps) are able to induce protective immune responses against pathogens and tumors after injection into immunocompetent hosts. The activation of components of the adaptive immune system, including cytotoxic T lymphocytes specific for pathogen- or tumor-derived peptides, is crucial for the establishment of immunoprotection. Hsps acquire these peptides during intracellular protein degradation and when released during necrotic cell death, facilitate their uptake and Minor Histocompatibility Complex (MHC)-restricted representation by professional antigen-presenting cells (APCs). In addition, the interaction of Hsps with APCs, including the Endoplasmatic Reticulum (ER)-resident chaperone glycoprotein 96 (Gp96), induces the maturation of these cells by Toll-like receptor (TLR)– mediated signaling events. We now provide evidence that in contrast to lipopolysaccharides (LPS)-mediated dendritic cell (DC) maturation, the interaction of Gp96 with DCs leads to the preferential expansion of antigen-specific CD8-positive T cells in vitro and in vivo. This CD8 preference induced by mouse and human DCs did not correlate with enhanced levels of interleukin-12 secretion. Thus, despite the fact that both LPS and Gp96 activate DCs in a TLR4-dependent manner, the experiments of this study clearly demonstrate qualitative differences in the outcome of this maturation process, which preferentially favors the expansion of CD8-positive T cells.
INTRODUCTION
Antigenic peptides chaperoned by heat shock proteins (Hsps) have been described as potent tumor vaccines in animal models and are currently studied in clinical trials. Recently, a considerable number of new functions have been uncovered, particularly for one Hsp, the Endoplasmatic Reticulum (ER)-resident glycoprotein 96 (Gp96, also known as glucose-regulated protein 94). Gp96 is not only a peptide carrier but also targets surface receptors on antigen-presenting cells (APCs), eg, CD91 for efficient delivery of its peptide cargo into the MHC class I antigen–processing pathway. This results in the receptor-mediated cross-presentation of the Gp96-associated peptides on Minor Histocompatibility Complex (MHC) class I molecules and activation of cytotoxic T lymphocytes (CTLs) (Arnold-Schild et al 1999; Wassenberg et al 1999; Binder et al 2000b; Singh-Jasuja et al 2000b). Furthermore, Gp96 simultaneously activates APCs such as dendritic cells (DCs) in vitro (Basu et al 2000; Singh-Jasuja et al 2000a) and in vivo (Binder et al 2000a), resulting in increased costimulatory activity and release of proinflammatory cytokines and nitric oxide (Panjwani et al 2002). We have also demonstrated that maturation of DCs by Gp96 requires the presence of Toll-like receptor (TLR) 2 and 4 (Vabulas et al 2002b). These results support the speculation that Hsps such as Gp96 function as local danger signals in response to cellular stress. This has been underlined by several observations: Gp96 is released during necrotic cell death and viral lysis but not after apoptosis (Basu et al 2000; Berwin et al 2001). Necrotic lysates from primary tumor tissue able to mature the DCs have been shown to be enriched of Hsps, and the amount of Hsps in the lysates was critical for the ability of DC maturation (Somersan et al 2001). At the same time, platelets efficiently bind Gp96, neutralizing its ability to activate DCs, a proposed regulatory mechanism confining the effective area of activating Hsps to the local tissue (Hilf et al 2002). The APC-activating function of Gp96 is presumably an intrinsic capability independent of the associated peptides (Baker-LePain et al 2002).
Previously, we have shown that DCs activated by Gp96 exhibit an enhanced T-cell stimulatory capacity, demonstrated by in vitro proliferation assays with allogeneic T cells (Singh-Jasuja et al 2000a). Investigating this phenomenon in more detail, we now find that human monocyte– derived and mouse bone marrow–derived dendritic cells (BMDCs) matured by Gp96 activate CD8+ cytotoxic T cells rather than CD4+ helper T cells in vitro as well as in vivo. On the other hand, DC maturation by lipopolysaccharides (LPS) shows a preference for the expansion of CD4+ T cells. We conclude that the interaction of Gp96 with DCs induces maturation signals, which qualitatively differ from those mediated by LPS, resulting in the induction of immune responses dominated by CD8 T cells.
MATERIALS AND METHODS
Mice
C57BL/6 (H2b, CD90.2+) mice were obtained from Charles River, Bar Harbor, ME, USA. Congenic C57BL/ 6J-IghaThy1aGpi1a (H2b, CD90.1+) mice were obtained from Jackson Laboratories, Wilmington, MA, USA. OT-I (Hogquist et al 1994) and OT-II (Barnden et al 1998) mice, which have a transgenic T-cell receptor for the H2-Kb–restricted SIINFEKL peptide derived from ovalbumin257–264 or for the H2-Ab–restricted ISQAVHAAHAEINEAGR peptide derived from ovalbumin323–339, respectively, and St42 mice (transgenic T-cell receptor for H2-Db–restricted Ad5-E1A234–243 peptide) were obtained from the animal facility of the Leiden University Medical Center (The Netherlands) (den Boer et al 2001).
Generation of DC
Mouse immature DCs were generated from bone marrow of C57BL/6 mice, according to standard protocols (Inaba et al 1992) in Iscove's Modified Dulbecco medium (IMDM; BioWhittaker, Verviers, Belgium) supplemented with 200 mM l-glutamine (GIBCO-BRL Life Technologies, Paisley, UK), 100 IU/mL penicillin-streptomycin (GIBCO-BRL), 10% Fetal Calf Serum (FCS) (PAA, Linz, Austria), and cytokines as indicated below. In brief, bone marrow cells were incubated with 150 U/mL granulocyte macrophage–colony stimulation factor (GM-CSF, PeproTech, London, UK) for 7 days with medium renewed every 2 days. Approximately 90–100% of all cells in the Fluorescence Activated Cell Sorting (FACS) gate used for monocytes were DCs determined by flow cytometry with antibodies (obtained from BD PharMingen, San Diego, CA, USA) to be CD11c+, CD14−, CD86low, and H2-Ab+. Human immature DCs were prepared from peripheral mononuclear blood cells (according to Bender et al 1996) in X-Vivo 15 medium (BioWhittaker, Walkersville, MD, USA) supplemented with 200 mM l-glutamine, 100 IU/mL penicillin-streptomycin, 1% human serum (Peel-Freez, Brown Deer, WI, USA), and cytokines as indicated below. In brief, monocytes isolated by Ficoll density gradient (Lymphoprep, Nycomed, Oslo, Norway) and plastic adherence were cultured in medium supplemented with 10 μg/mL interleukin-4 (IL-4, R&D Systems, MN, USA) and 50 μg/mL GM-CSF (Leukomax, Novartis Pharma GmbH, Nürnberg, Germany) for 6–8 days. The cells generated in this way showed a large number of dendrites up to day 12 and were only slightly adherent. They expressed CD1a, low CD14, low CD86, Human Leukocyte Antigen (HLA)-DR, and very low CD83 on their surface, as determined by different antibodies (from BD PharMingen, San Diego, CA, USA) in flow cytometry (data not shown). All FACS analyses were performed on a FACSCalibur® (BD PharMingen, Mountain View, CA, USA) by Cell Quest Software.
Stimulation of DCs
Mouse BMDCs were stimulated by addition of Gp96 or heat-treated Gp96 (95°C for 20 minutes) or LPS (from Salmonella typhimurium, Sigma Chemical Co., St Louis, MO, USA) for 6 hours to 3 days. Gp96 was used at 100 μg/ mL and LPS at 100 ng/mL unless indicated otherwise. Gp96 (kindly provided by Immatics Biotechnologies, Tü bingen, Germany) was purified from the IGELa2 mouse cell line (tested mycoplasma free). Endotoxin content in commercial Gp96 preparations was tested using a Limulus Amebocyte Lysate Kit (QCL-1000, BioWhittaker), according to the guidelines published by the US Food and Drug Administration. The endotoxin content determined in all cases was below 0.05 EU/μg Gp96.
Supernatants were taken after 18–24 hours, and mouse IL-12 (p40) was measured using standard sandwich enzyme-linked immunosorbent assay (ELISA) protocols (antibodies and standards by BD PharMingen), streptavidin-conjugated horseradish peroxidase, and 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) substrate (Sigma). Furthermore, on day 3 after activation, expression of the costimulatory molecule CD86 was measured by flow cytometry (data not shown).
Human monocyte–derived, immature DCs (day 7) were stimulated by Gp96, heat-treated Gp96, LPS, or heat-treated LPS. Gp96 was used at 100 μg/mL and LPS at 100 ng/mL unless indicated otherwise. Supernatants were assayed for tumor necrosis factor (TNF)–α or IL-12 by sandwich ELISA as described above (data not shown). On day 3 after activation, expression of costimulatory molecules CD83 and CD86 and MICA/B were measured by flow cytometry.
Stimulation of alloreactive T cells
Human DCs were stimulated with different activators in a 96-well plate for 3 days, as described above, washed extensively, and incubated with peripheral blood lymphocytes (PBLs) (ratio 1:10) from a different donor for 5 days. CD69 and MHC class II (data not shown) expression, both markers for human T-cell activation, was determined on day 1 or 5, respectively, by flow cytometry.
Stimulation of St42 CD8 T cells in vitro
Immature BMDCs were activated, as described above, for 3 days, washed extensively, and loaded with 1 μg/mL H2-Db–restricted Ad5-E1A234–243 peptide (sequence SGPSNTPPEI) for 1.5 hours at 37°C. E1A peptide was synthesized on an ABI 432A peptide synthesizer (Applied Biosystems) applying Fmoc chemistry; 2 × 104 peptide–loaded BMDCs were washed 4 times and incubated with 1 × 106 carboxyfluorescein diacetate succinimide ester (CFSE)–labeled splenocytes from St42 mice. Four and 5 days later, proliferation and intracellular interferon (IFN)-γ production were measured by flow cytometry. Cells were labeled with CFSE by incubation with 1 μM CFSE in phosphate-buffered saline (PBS) at room temperature for 3 minutes in the dark and then washed 3 times with IMDM-containing 10% FCS. For flow cytometric analysis, intracellular IFN-γ staining was performed using the Cytofix/Cytoperm™ kit (BD Pharmingen) according to the instructions of the manufacturer using a phycoerythrin-labeled anti-mouse IFN-γ antibody (BD Pharmingen).
Adoptive transfer
St42 adoptive transfer
A total of 30 × 106 CFSE-labeled CD90.1+ heterozygous St42 spleen cells were injected intravenously (i.v.) into CD90.2+ homozygous C57BL/6 on day 0. Transferred CSFE-labeled cells could be detected by flow cytometry in peripheral blood on day 1. On day 2, BMDCs from C57BL/6 mice activated with different stimulators, as described above, were loaded with 100 μg/mL H2-Db–restricted Ad5-E1A234–243 peptide or 100 μg/mL H2-Db–restricted Ad5-E1B peptide (sequence VNIRNCCYI) as a negative control peptide for 1 hour at 37°C, and 4 × 105 peptide–loaded BMDCs were injected intraperitoneally (i.p.) into the mice. At days 6 and 8, cells from peripheral blood as well as lymph nodes were analyzed by flow cytometry.
OT-I/OT-II adoptive transfer
OT-I/OT-II adoptive transfer was performed similar to the adoptive transfer of St42 mice except for the following changes: 30 × 106 CFSE-labeled CD90.2+ homozygous OT-I or OT-II spleen cells were injected i.v. into CD90.1+ homozygous C57BL/6 mice on day 0. BMDCs from C57BL/6 mice were incubated with 100 ng/mL H2-Kb– restricted ova257–264 peptide or 10 μg/mL H2-Ab–restricted Ova323–339 peptide for 1 hour at 37°C.
RESULTS
Dendritic cells are activated by Gp96
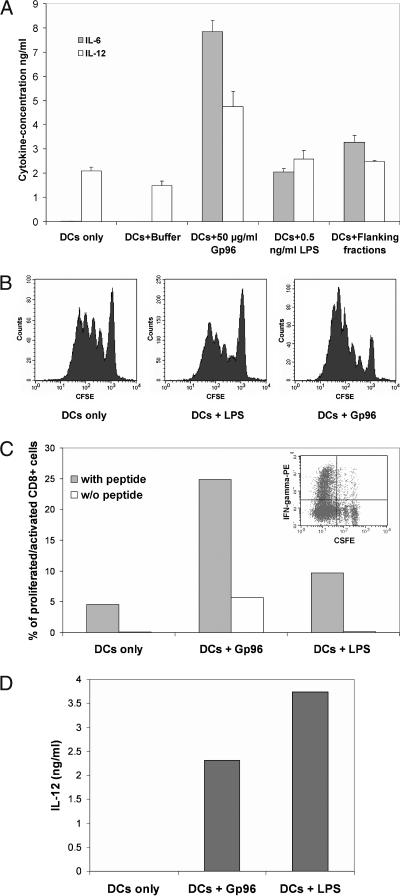
The BMDCs were generated from C57BL/6 mice according to established protocols. Incubation of these immature DCs with Gp96 for 3 days led to the upregulation of the costimulatory molecules CD80 and CD86 (data not shown, see [Singh-Jasuja et al 2000a]) and the release of proinflammatory cytokines such as IL-12 and IL-6 (Fig 1A). Because the LPS from gram-negative bacteria are also described as potent TLR4-dependent DC stimulators, the possibility of LPS contaminations in the Gp96 preparation had to be addressed. For this, the following control experiments were performed. (1) Both Gp96 and LPS were boiled for 20 minutes before addition to the cell culture medium. Although the activity of Gp96 was completely lost by the heat treatment, the activity of LPS was not affected (Singh-Jasuja et al 2000a). (2) The addition of the endocytosis inhibitor monodansylcadaverine (MDC) abolished the ability of Gp96 but not of LPS to induce DC activation. Thus, the activation of DCs by Gp96 depends on the endocytosis of Gp96, whereas activation by LPS is endocytosis independent (Vabulas et al 2002b). (3) Endotoxin levels in the Gp96 preparations by Limulus lysate assay were determined to be below 0.05 EU/μg. Low amounts of LPS (0.5 ng/mL), which correspond to the level of endotoxin detected in Gp96 preparations, were not able to mature DCs (Fig 1A). (4) Fractions obtained from the chromatographic purification of Gp96, which contained no Gp96 but similar levels of endotoxin when compared with the Gp96-containing fraction, did not lead to maturation of DCs (Fig 1A).
Fig 1.
Mouse bone marrow–derived dendritic cells (BMDCs) matured by glycoprotein 96 (Gp96) induce peptide-specific proliferation and interferon (IFN)-γ production by CD8 T cells in vitro. (A) Cytokine production by mouse BMDCs matured by Gp96 or control stimuli for 3 days. (B) Proliferation of St42 CD8 T cells in vitro. BMDCs from C57BL/6 mice were treated with Gp96 or lipopolysaccharides for 3 days and loaded with Ad5-E1A peptide to be used for coculture with carboxyfluorescein diacetate succinimide ester–labeled spleen cells from St42 mice. Proliferation of CD8 T cells was assayed by flow cytometric analysis on day 4 after T-cell stimulation. (C) IFN-γ production of St42 CD8 T cells in vitro. The graph shows the percentage of intracellular IFN-γ production of the most proliferated CD8 T cells on day 5 (see insert). (D) Interleukin-12 production of the BMDCs used for the in vitro activation of St42 T cells. The results are representative of 3 independent experiments
DCs matured by Gp96 show enhanced stimulation of peptide-specific CD8 T cells in vitro
To study the activation of peptide-specific T cells, BMDCs from C57BL/6 mice were activated by Gp96, boiled Gp96 (both at 100 μg/mL), or LPS at 100 ng/mL. These concentrations were selected because they induce optimal BMDC activation, as judged by the expression of CD80 and CD86 and the production of the proinflammatory cytokines IL-6, IL-12, and TNF-α (data not shown). One day later, the activation status of the BMDCs was measured by determining IL-12 levels in the supernatant (Fig 1D). Three days after activation, these DCs were loaded with E1A peptide, washed extensively, and cocultured with CFSE-labeled spleen cells from St42 mice expressing transgenic T-cell receptors specific for E1A peptide bound to H2-Db molecules. The fluorescein-based dye, CFSE is split among daughter cells during cell division; thus, the emission of CFSE-labeled cells is reduced with every round of proliferation (Weston and Parish 1990). Four days after T-cell stimulation, proliferation of CD8 T cells was measured by determining the level of CFSE (Fig 1B). On day 5, activation (by determining intracellular IFN-γ production) and proliferation of CD8 T cells in the culture were measured simultaneously (Fig 1C). Thus, DCs matured by Gp96 induce a significantly stronger proliferation (Fig 1B) as well as activation (Fig 1C) of CD8 T cells from St42 mice compared with LPS-matured DCs. This was observed despite the fact that LPS-matured DCs produced higher levels of IL-12 (Fig 1D) or IL-6 and showed no difference in the expression of costimulatory molecules (data not shown).
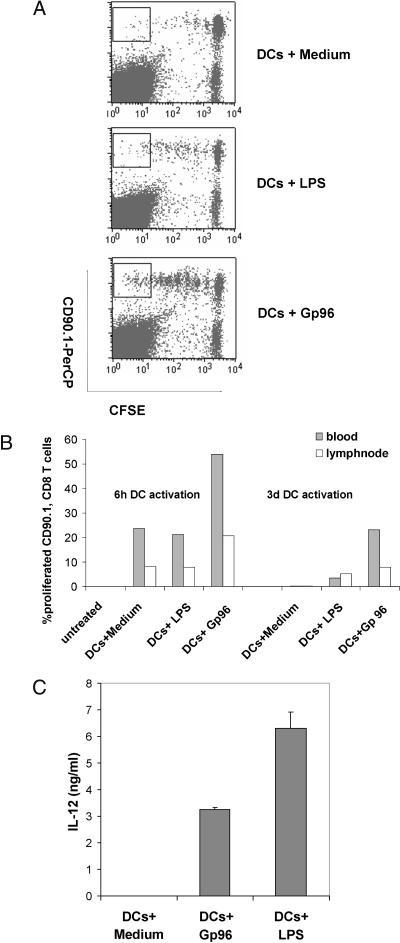
Gp96-matured DCs show enhanced stimulation of CD8 T cells in vivo
Next, we extended the results obtained for the proliferation of St42 T cells in vitro to an in vivo model. For this, CFSE-labeled spleen cells from St42 mice (CD90.1+) were injected into the tail vein (i.v.) of CD90.2+ C57BL/6 mice. Twenty-four hours later, the injected CD90.1+ CFSE-labeled St42 T cells could be detected in the blood of CD90.2+ mice and represented approximately 1% of all peripheral blood cells (data not shown). One day later, BMDCs matured by Gp96 or LPS for 6 hours or 3 days were loaded with E1A peptide or irrelevant E1B control peptide and injected i.p. into these mice. Eight days after injection of St42 cells, peripheral blood and draining lymph nodes were collected to analyze the proliferation of the CFSE-labeled CD90.1+ St42 T cells in response to DC immunization. As shown in Figure 2, in vivo proliferation of St42 T cells in response to BMDCs matured by Gp96 was significantly stronger compared with nonmatured or LPS-matured DCs (Fig 2 A,B), although Gp96- and LPS-induced maturation of DCs was comparable, as judged on the basis of IL-12 (Fig 2C) or IL-6 production determined before DC injection. No proliferation of St42 T cells was observed without injection of DCs (Fig 2B) or when DCs loaded with an irrelevant control peptide were injected (data not shown).
Fig 2.
Bone marrow–derived dendritic cells (BMDCs) matured by glycoprotein 96 (Gp96) induce peptide-specific proliferation of cytotoxic T lymphocytes in vivo. On day 0, carboxyfluorescein diacetate succinimide ester –labeled St42 spleen cells (CD90.1) were injected i.v. into C57BL/6 mice (CD90.2). BMDCs from C57BL/6 mice were matured by Gp96 or lipopolysaccharides (LPS) for 6 hours or 3 days and loaded with Ad5-E1A peptide or Ad5-E1B peptide as a negative control. BMDCs were injected intraperitoneally into C57BL/6 mice previously transferred with St42 splenocytes. On day 8, peripheral blood samples and draining lymph nodes were collected. (A) Proliferation of St42 CD8 T cells from lymph nodes 6 days after immunization with Ad5-E1A peptide–loaded dendritic cells matured by Gp96 or LPS for 3 days. (B) Percentage of proliferated St42 CD8 T cells (gate as shown in [A]) after immunization with peptide-loaded BMDCs stimulated with Gp96 or LPS for 6 hours or 3 days as indicated. Untreated mice received St42 spleen cells only. (C) Interleukin-12 production of BMDCs stimulated with Gp96 or LPS and used for immunization. Error bars give SD of triplicates. The results are representative of 2 independent experiments
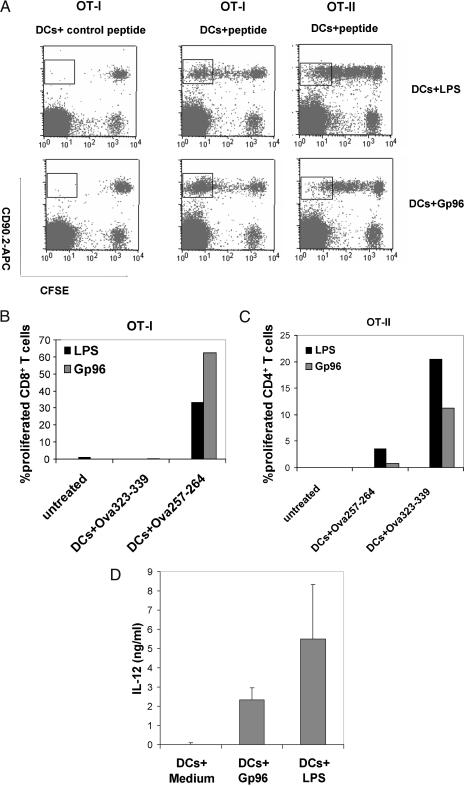
Mouse Gp96-matured DCs preferentially activate CD8 T cells vs CD4 T cells in vivo
Although Gp96 and LPS were able to mature BMDCs in vitro to a similar extent, Gp96-matured DCs induced a stronger CD8 T cell activation compared with LPS-matured DCs. To directly compare the effects of DCs matured by Gp96 or LPS on the proliferation of CD8 and CD4 T cells as well, the OT-I and OT-II transgenic mouse systems were selected. T cells from OT-I mice express a transgenic T-cell receptor specific for an ovalbumin-derived MHC class I epitope, whereas OT-II transgenic T cells recognize an MHC class II epitope from the same protein. Analogous to the experiments above using T cells from St42 mice, CFSE-labeled OT-I or OT-II spleen cells (CD90.2+) were injected i.v. into C57BL/6 mice (CD90.1+). Forty-eight hours later, Gp96- or LPS-matured BMDCs, which were loaded with either the MHC class I– or the MHC class II–restricted peptide from ovalbumin, were injected i.p. into these mice. On day 8, peripheral blood and lymph nodes were collected to analyze the proliferation of CFSE-labeled CD90.2+ OT-I or OT-II cells. Although DCs were activated to similar levels by Gp96 or LPS as judged by the production of IL-12 in vitro (Fig 3D), proliferation of the CD8+ OT-I T cells was significantly stronger when Gp96-matured DCs were used in comparison with LPS-matured DCs (Fig 3 A,B). On the other hand, proliferation of CD4+ OT-II T cells was more pronounced when LPS-matured DCs were used as stimulator cells (Fig 3 A,C). Loading of DCs with a control peptide (Fig 3 A–C) or injection of PBS (Fig 3 B,C) did not result in cell proliferation.
Fig 3.
Mouse glycoprotein 96 (Gp96)-matured dendritic cells (DCs) preferentially activate CD8 vs CD4 T cells in vivo. On day 0, carboxyfluorescein diacetate succinimide ester –labeled OT-I or OT-II spleen cells (CD90.2+) were injected i.v. into C57BL/6 mice (CD90.1+). On day 2, bone marrow–derived dendritic cells from C57BL/6 mice matured by Gp96 or lipopolysaccharides (LPS) for 6 hours were loaded with H2-Kb–restricted Ova257–264 peptide or H2-Ab–restricted Ova323–339 peptide and injected intraperitoneally into C57BL/6 mice previously injected with OT-I or OT-II spleen cells. On day 8, peripheral blood samples and draining lymph nodes were collected. (A) Proliferation of OT-I or OT-II T cells in lymph nodes. (B, C) Summary of the proliferation of OT-I and OT-II T cells in vivo. The graphs show the percentage of proliferation present in the gates shown in (A). Untreated mice received OT-I or OT-II spleen cells only. (D) Interleukin-12 production of DCs matured by Gp96 and LPS. Error bars give SD of triplicates. The results are representative of 2 independent experiments
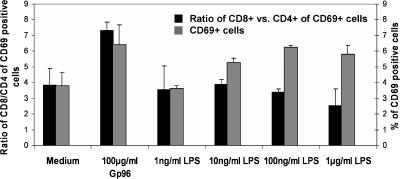
Human Gp96-matured DCs preferentially stimulate CD8 T cells
Next, the preference for CD8 T-cell activation by Gp96-matured DCs was analyzed using human cells. Monocyte-derived human DCs were generated from buffy coats according to established protocols by plastic adherence and culturing of the purified monocytes with GM-CSF and IL-4 for 7 days. Incubation of these immature DCs with Gp96 or LPS for 3 days led to the upregulation of the costimulatory molecule CD86 and the maturation marker CD83 as well as to the release of proinflammatory cytokines such as IL-12, TNF-α, and IL-6 (data not shown). DCs activated by Gp96 or LPS at the concentrations indicated were cocultured with allogeneic PBL. Activation of the T cells was determined by the analysis of the activation markers CD69 on day 1 (Fig 4) and MHC class II molecules on day 5 (data not shown) after DC coculture. As expected, significantly less T cells were activated by immature DCs (“Medium”) compared with DCs matured by Gp96 or LPS (see also Singh-Jasuja et al 2000a). When analyzing the expansion of CD8+ vs CD4+ T cells, we observed that Gp96-matured DCs preferentially activated CD8+ T cells. This preference was not observed when LPS was used for the maturation of DCs (Fig 4). Reducing the amount of LPS or Gp96 resulted in a reduced overall T-cell activation as evident from the lower numbers of CD69+ T cells but did not influence the ratio of CD8+ vs CD4+ T cells (Fig 4 and data not shown).
Fig 4.
Human dendritic cells (DCs) matured by glycoprotein 96 (Gp96) preferentially induce activation of allogeneic CD8 T cells. Monocyte-derived human dendritic cells were incubated with Gp96 or lipopolysaccharides at the indicated concentrations for 3 days and then cocultured with allogeneic peripheral blood lymphocyte. The graph shows the percentage of CD69-expressing T cells (gray bars) and the CD8/CD4 ratio of CD69-positive T cells (black bars) 1 day after stimulation with matured DCs. Results are representative of 2 independent experiments
DISCUSSION
Hsps such as Gp96 and Hsp70 have been shown to promote the maturation of BMDCs in vivo and in vitro (Todryk et al 1999; Basu et al 2000; Singh-Jasuja et al 2000a) and to contribute to CTL activation (Srivastava et al 1998; Schild et al 1999; Schild and Rammensee 2000; Srivastava 2002). This results in the secretion of proinflammatory cytokines (Fig 1A) and the upregulation of costimulatory molecules in a TLR2/4-dependent fashion (Asea et al 2002; Vabulas et al 2002a, 2002b).
In this study, we analyzed whether BMDC activation by Gp96 induces adaptive immune responses, which differ from those induced by LPS-activated BMDCs. The reason to compare these 2 stimuli is based on the fact that LPS is present during the infections with gram-negative bacteria, which will be controlled predominantly by antibody-dominated immune responses. Gp96, on the other hand, is released during necrotic cell death, for example, as a consequence of viral infections (Berwin et al 2001); the elimination of many viruses requires the activation of CTLs.
Using BMDCs activated with either of the 2 stimuli, we find that Gp96-mediated stimulation promotes predominantly the activation and expansion of antigen-specific CD8+ T cells in vitro (Fig 1 B,C) and in vivo (Figs 2 and 3), whereas LPS-mediated stimulation favors the activation and expansion of antigen-specific CD4+ T cells (Fig 3). This CD8-biased T cell response cannot account for the production of IL-6 and IL-12 and the expression levels of MHC and costimulatory molecules (Figs 1D, 2C, 3D; data not shown), since Gp96- or LPS-stimulated BMDCs do not differ significantly in this activation-induced expression profile. A similar observation is made for the activation of alloreactive CD8+ T cells by human DCs stimulated by Gp96. Examining the ratio of activated CD8+ vs CD4+ T cells, we find that Gp96-activated human DCs unlike LPS-activated DCs preferentially stimulate the activation of CD8+ T cells. The reduced activation of CD8+ T cells by LPS-activated DCs does not depend on the amount of LPS used for DC activation. Varying the concentration of LPS changes only the number of CD69+ activated T cells but does not affect the ratio of CD8+ vs CD4+ T cells (Fig 4).
Our findings suggest qualitative differences in the activation of DCs by Gp96 or LPS. However, the direct or indirect molecular mechanisms or interactions between Gp96-matured DCs and T cells responsible for this preference remain to be determined. We have compared the secretion of several proinflammatory cytokines, the upregulation of various costimulatory molecules including CD80, CD86, CD137L (4–1BB ligand), the upregulation of MHC class I vs class II molecules, and the induction of MICA/B molecules, which are known to interact with NKG2D molecules present on the surface of CD8+ but not CD4+ T cells (Bauer et al 1999) (data not shown). All these molecules are not expressed at significantly different levels between Gp96- and LPS-matured DCs and are therefore unlikely to provide an explanation for our findings.
The previously reported participation of TLR4 in APC activation by Hsps initiated discussions about the contribution of endotoxin contaminations to the observed effects. Several arguments for an endotoxin-independent activity of Hsps have been discussed previously. They include that Hsp effects were sensitive to heat treatment, the presence of MDC and, in addition, were insensitive to polymyxin B (Singh-Jasuja et al 2000a; Zheng et al 2001; Baker-LePain et al 2002). Furthermore, a study by Reed et al (2003) demonstrated that highly purified Gp96 molecules with almost undetectable endotoxin contaminations (<0.027 pg/μg Gp96) are still able to promote phosphorylation of Extracellular-signal regulated kinase (ERK), which is part of the activation pathway preceding proinflammatory cytokine secretion. Nevertheless, experiments using recombinant Hsp70 molecules suggested that endotoxin contaminations are exclusively responsible for the APC-activating capabilities of these Hsp (Bausinger et al 2002; Gao and Tsan 2003). However, the experiments presented in this study provide additional evidence that endotoxin contaminations present in the Hsp preparations are not exclusively responsible for the activation of DCs. One attractive hypothesis that would provide an explanation for this controversy is the possibility that LPS interacts with Hsps as shown recently for Gp96 (Reed et al 2003) or Hsp60 (Habich et al 2005) and that this interaction modulates or augments the biological effects of low amounts of LPS present in Gp96 preparations, which on their own might be unable to induce DC activation.
In summary, our data show that Hsps such as Gp96 are molecules, which are able to participate in the activation of cells of the innate immune system and thereby influence the outcome of adaptive immune responses.
Acknowledgments
The synthetic peptides for this work were kindly provided by Stefan Stevanovic (Tübingen). This work was supported by grants of the Deutsche Forschungsgemeinschaft (Leibnizprogram to H.-G. Rammensee [Ra369/4-1], Graduiertenkolleg 794/1 and SFB432, B10 to H. Schild).
Footnotes
Sabina Rayo Ramirez, Harpreet Singh-Jasuja, and Tobias Warger contributed equally to this study.
REFERENCES
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, Schild H. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760.0022-1767(1999)162[3757:CEREOH]2.0.CO;2 [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200.0021-9258(2002)277[15028:NSTPUB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med. 2002;196:1447–1459. doi: 10.1084/jem.20020436.0022-1007(2002)196[1447:GGAGNG]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x.0818-9641(1998)076[0034:DTEITM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappaB pathway [In Process Citation] Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539.0953-8178(2000)012[1539:NBNACD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727.0193-4511(1999)285[0727:AONCAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bausinger H, Lipsker D, and Ziylan U. et al. 2002 Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur J Immunol. 32:3708–3713. [DOI] [PubMed] [Google Scholar]
- Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8.0022-1759(1996)196[0121:IMFTGO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Berwin B, Reed RC, Nicchitta CV. Virally induced lytic cell death elicits the release of immunogenic GRP94/gp96. J Biol Chem. 2001;276:21083–21088. doi: 10.1074/jbc.M101836200.0021-9258(2001)276[21083:VILCDE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c(+) cells in vivo. J Immunol. 2000a;165:6029–6035. doi: 10.4049/jimmunol.165.11.6029.0022-1767(2000)165[6029:CEHSPG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000b;1:151–155. doi: 10.1038/77835.1529-2908(2000)001[0151:CARFHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- den Boer AT, Diehl L, and van Mierlo GJ. et al. 2001 Longevity of antigen presentation and activation status of APC are decisive factors in the balance between CTL immunity versus tolerance. J Immunol. 167:2522–2528. [DOI] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200.0021-9258(2003)278[0174:ECIRHH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Habich C, Kempe K, van der ZR, Rumenapf R, Akiyama H, Kolb H, Burkart V. Heat shock protein 60: specific binding of lipopolysaccharide. J Immunol. 2005;174:1298–1305. doi: 10.4049/jimmunol.174.3.1298.0022-1767(2005)174[1298:HSPSBO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hilf N, Singh-Jasuja H, Schwarzmaier P, Gouttefangeas C, Rammensee HG, Schild H. Human platelets express heat shock protein receptors and regulate dendritic cell maturation. Blood. 2002;99:3676–3682. doi: 10.1182/blood.v99.10.3676.0006-4971(2002)099[3676:HPEHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4.0092-8674(1994)076[0017:TCRAPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693.0022-1007(1992)176[1693:GOLNOD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168:2997–3003. doi: 10.4049/jimmunol.168.6.2997.0022-1767(2002)168[2997:HSPGAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Reed RC, Berwin B, Baker JP, Nicchitta CV. GRP94/gp96 elicits ERK activation in murine macrophages: a role for endotoxin contamination in NF-{κ}B activation and nitric oxide production. J Biol Chem. 2003;278:31853–31860. doi: 10.1074/jbc.M305480200.0021-9258(2003)278[31853:GEEAIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schild H, Arnold-Schild D, Lammert E, Rammensee HG. Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr Opin Immunol. 1999;11:109–113. doi: 10.1016/s0952-7915(99)80019-3.0952-7915(1999)011[0109:SPAIMB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schild H, Rammensee HG. gp96—the immune system's Swiss army knife. Nat Immunol. 2000;1:100–101. doi: 10.1038/77770.1529-2908(2000)001[0100:GISSAK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes REM, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000a;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0.0014-2980(2000)030[2211:THSPGI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H, Toes RE, and Spee P. et al. 2000b Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 191:1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167:4844–4852. doi: 10.4049/jimmunol.167.9.4844.0022-1767(2001)167[4844:PTTLAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749.1474-1733(2002)002[0185:ROHPII]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1.1074-7613(1998)008[0657:HSPCOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408.0022-1767(1999)163[1398:HSPIDT]2.0.CO;2 [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/ interleukin-1 receptor signal pathway. J Biol Chem. 2002a;277:15107–15112. doi: 10.1074/jbc.M111204200.0021-9258(2002)277[15107:HAESOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Braedel S, and Hilf N. et al. 2002b The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 277:20847–20853. [DOI] [PubMed] [Google Scholar]
- Wassenberg JJ, Dezfulian C, Nicchitta CV. Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J Cell Sci. 1999;112:2167–2175. doi: 10.1242/jcs.112.13.2167.0021-9533(1999)112[2167:RMAFPP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m.0022-1759(1990)133[0087:NFDFLM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731–6735. doi: 10.4049/jimmunol.167.12.6731.0022-1767(2001)167[6731:CSTOHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]