Abstract
“French type” sialuria, a presumably dominant disorder that, until now, had been documented in only five patients, manifests with mildly coarse facies, slight motor delay, and urinary excretion of large quantities (>1 g/d) of free N-acetylneuraminic acid (NeuAc). The basic defect consists of the very rare occurrence of failed feedback inhibition of a rate-limiting enzyme, in this case uridinediphosphate-N-acetylglucosamine (UDP-GlcNAc) 2-epimerase, by a downstream product, in this case cytidine monophosphate (CMP)–NeuAc. We report a new patient with sialuria who has a heterozygous G→A substitution in nucleotide 848 of the epimerase gene, which results in an R266Q change. The proband’s other allele, as expected, had no mutation. However, the heterozygous R266Q mutation was detected in the patient’s mother, who has similarly increased urinary levels of free NeuAc, thereby confirming, for the first time, the dominant mode of inheritance of this inborn error. The biochemical diagnosis of the proband was verified by the greatly increased level of free NeuAc in his cultured fibroblasts, the NeuAc distribution, mainly (59%) in the cytoplasm, and by the complete failure of 100 μM CMP-NeuAc to inhibit UDP-GlcNAc 2-epimerase activity in the mutant cells. These findings call for expansion of the phenotype to include adults and for more-extensive assaying of free NeuAc in the urine of children with mild developmental delay. The prevalence of sialuria is probably grossly underestimated.
Introduction
“French type” sialuria (MIM 269921) is a rare human metabolic disorder caused by failed feedback inhibition of a catalytically normal enzyme (Weiss et al. 1989; Seppala et al. 1991). The enzyme is uridinediphosphate-N-acetylglucosamine (UDP-GlcNAc) 2-epimerase, which is rate limiting in the synthesis of sialic acid or N-acetylneuraminic acid (NeuAc). The allosteric feedback inhibitor is cytidine monophosphate-N-acetylneuraminic acid (CMP-Neu5Ac) (Kornfeld et al. 1964; Sommar and Ellis 1972), which also donates sialic acid for the synthesis of sialylglycoconjugates (Aula and Gahl 2001). The lack of feedback inhibition of UDP-GlcNAc 2-epimerase results in vastly increased urinary excretion of free NeuAc (Thomas et al. 1985; Gahl et al. 1996). Although the first patient with sialuria was reported in France >30 years ago (Fontaine et al. 1968; Montreuil et al. 1968), molecular characterization of this type of sialuria was only recently accomplished (Seppala et al. 1999). Because an entirely normal coding sequence was demonstrated in one allele of each patient investigated, sialuria was proposed to be inherited in a dominant fashion.
To date, five patients have been reported with sialuria (Fontaine et al. 1968; Wilcken et al. 1987; Seppala et al. 1991; Krasnewich et al. 1993; Ferreira et al. 1999); most have mild developmental delay and coarse facial features but no other physical signs of a storage disorder. In the present report, we provide a detailed clinical, biochemical, and molecular report of the sixth (Leroy et al. 1998) and seventh patients with sialuria, a boy and his mother. These patients confirm, for the first time, the autosomal dominant nature of the disorder and expand the clinical phenotype of this inborn error. The results indicate that the prevalence of sialuria may be grossly underestimated and that diagnostic evaluations of appropriate individuals should be performed more frequently.
Subjects and Methods
Clinical Reports
Patient 1
The boy was born, after 35 weeks of gestation, to an unaffected 30-year-old woman, by normal vertex delivery. The 32-year-old father was unrelated to the mother. Two older brothers were unaffected. Birth length was 44 cm, and occipitofrontal circumference (OFC) was 33 cm. Because of mild grunting, hypotonia, and low birth weight (2,350 g), the newborn infant spent one week in an incubator and received oxygen. He had prolonged mild jaundice. At 2 mo of age, frequent opisthotonic posturing and persistent hypotonia were noticed. Anemia was treated with a transfusion of packed red blood cells. At 3 mo of age, a CT scan of the brain showed a widened interhemispheric fissure and widened cortical sulci but did not show any anatomic or textural abnormalities. Excessive rhinorrhea and recurrent respiratory infections were present throughout infancy.
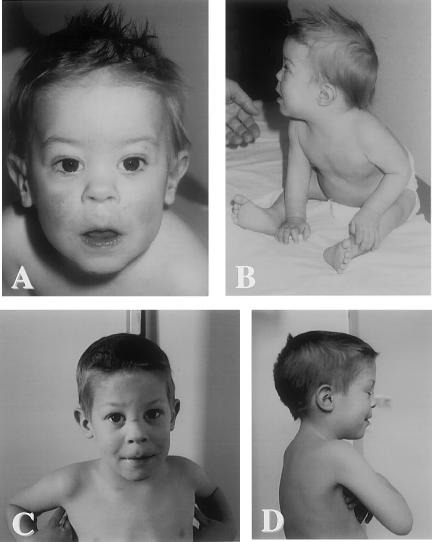
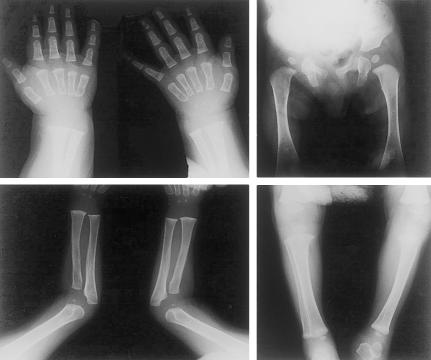
At 10 mo of age, failure to thrive (FTT) was documented, and a storage disorder was suspected because of poor growth, coarse facial features, and a new viral infection with dehydration that required hospital admission. Weight and length were 7,340 g and 68 cm, respectively, and the OFC was 45.5 cm. The patient remained hypotonic but was alert and grasped objects. He crawled and was able to sit and stand with support. The head was scaphocephalic, and the facies was flat and plump. There was mild proptosis of the eyes, a low nasal bridge, and epicanthal folds (fig. 1A and 1B). There was no organomegaly or lymphadenopathy. The range of motion was full in all joints, including the shoulders. Deep-tendon reflexes were symmetrically weak. Radiographs showed mildly shortened and broad diaphyses of long bones, squat pubic and ischial bones, and slightly shortened and widened osteopenic metacarpals (fig. 2). The skeletal age was between 3 and 6 mo.
Figure 1.
Clinical features in patient 1. A and B, Proband at age 10.5 mo. Note flat, mildly coarse facies, epicanthal folds, and low nasal bridge; excessive nasal discharge; difficulty sitting without support; and mild axial hypotonia. C and D, Proband at age 4 years. The patient is considerably improved, with significant neuromotor progress. Note accidental scar on nose, absence of facial coarsening, and no limitation of motion in any joints.
Figure 2.
Skeletal radiographs of proband at age 10.5 mo. Note slightly short and wide long-bone diaphyses; squat pubic and ischial bones; osteopenic, mildly short, and coarsely trabeculated metacarpals; and widened metaphyses in the distal ulnae and radii, distal femora, and proximal tibiae. The skeletal age was between 3 and 6 mo.
Results of routine urine screening were normal for glycosaminoglycans and neutral and charged oligosaccharides. Erythrocyte and leukocyte counts (total and differential) were normal. Frank microcytic hypochromic anemia was successfully treated with a transfusion of packed red blood cells. Results of thyroid-function tests in serum were within normal limits. The specific activities of several lysosomal acid hydrolases in serum were either normal or two to three times the value in age-matched controls. The corresponding activities in leukocytes and fibroblasts were normal, but the activity of β-d-galactosidase was double that in controls. Urinary concentration of free sialic acid was 14,680 μmol/mmol creatinine (control <74 μmol/mmol). A skin biopsy was performed.
Impaired hip and knee extensions were noted at age 15 mo. The boy remained hypotonic but alert and physically active. At 32 mo, his neuromotor and physical development had progressed significantly, and his weight was 12 kg (3d–10th percentile), his length was 93 cm (25th–50th percentile), and his OFC was 49.9 cm (25th–50th percentile). The liver edge was felt 2 cm below the costal margin. He had started walking alone at 2 years of age but maintained a broadly based gait. He spoke several words but made no two-word sentences. Grasping remained primitive, and social contacts were elementary. The boy was briefly admitted to hospital for a tonsillectomy and adenoidectomy. The enlarged tonsils, described as unusually pale, were examined histologically. At age 38 mo, a psychometric evaluation—using the McCarthy Scales of Children’s Abilities, the motor part of the Bayley Scales of Infant Development, and the Raynell Language Scale—yielded a general cognitive index of 70, which is consistent with borderline mental handicap that manifested primarily as delayed development of language and motor skills.
When the proband was seen shortly after his fourth birthday, his general health had improved considerably. He weighed 15 kg (∼25%). His height was 99.3 cm (10th–25th percentile), and his OFC was 50.4 cm (∼50 percentile) (fig. 1C and 1D). There was no organomegaly, and respiratory infections had become mild and infrequent. The lumbar spine was slightly hyperlordotic and was associated with minimal flexion contracture in both hips. The knees were held in mild flexion. No neurologic abnormality was found. The skeletal age was between 2.5 and 3 years. At age 4 years and 8 mo the urinary level of free NeuAc was 8,950 mmol/mmol creatinine.
Patient 2
This 34-year-old woman, the mother of patient 1, was a healthy homemaker of normal stature without dysmorphic features. Like her sole brother, she had completed only regular grade school. In contrast, all four of her sisters graduated from various college-level training programs. The patient briefly held domestic employment before marriage. Additional objective data on her early-childhood development, neuromotor status, and results of psychometric testing were not available. After patient 2 was found to manifest the same UDP-GlcNAc 2-epimerase gene mutation as her son, her urinary level of free NeuAc was found to be 4,278 μmol/mmol creatinine (control <74 μmol/mmol) or 48 μmol/mg creatinine. The urinary levels of free NeuAc in the father of patient 1, in the father and all five sibs of patient 2, and in the two older brothers of patient 1 were normal. The maternal grandmother had died of liver cancer at the age of 49 years.
Cellular and Biochemical Studies
Fibroblasts were cultured, and their subcellular fractions were prepared as described elsewhere (Seppala et al. 1991). Assay of free NeuAc in urine and whole-cell homogenates was performed according to the method of Cardo et al. (1985). For analysis of subcellular fractions and lymphoid tissue, free NeuAc was determined using anion-exchange chromatography with a Dionex AS6-Ion-Pack column, followed by quantitation using pulsed amperometric detection (Dionex Bio), as described elsewhere (Seppala et al. 1991). Specific activities of lysosomal enzymes in plasma, leukocytes, and fibroblasts were determined as reported elsewhere (Leroy et al. 1972). UDP-GlcNAc 2-epimerase activity and the degree of inhibition by CMP-sialic acid were studied by methods published elsewhere (Weiss et al. 1989; Seppala et al. 1991).
Molecular Investigations
Total RNA was extracted from cultured skin fibroblasts, using Trizol reagent (Life Technologies), and was reverse transcribed to cDNA, using the Superscript First Strand Synthesis System (Life Technologies). The patient’s cDNA was screened for mutations in the epimerase gene as described by Seppala et al. (1999). Nucleotide numbering is according to GenBank accession number NM_005476. To confirm the heterozygous G→A (R266Q) mutation at nucleotide 848, genomic DNA was isolated from the blood of the proband and both his parents, using standard techniques (Sambrook et al. 1989). PCR amplification was employed to obtain a 385-bp fragment that included the mutation site. The forward PCR primer (5′-TGAGTTCCTAGATGAGTGAAG-3′) was located in the intron between cDNA nucleotides 818 and 819, and the reverse PCR primer (5′-CAGGTTGATCACAGGTGTT-3′) was located in the exon (cDNA nucleotides 1005–987) that contains the mutation site. PCR amplifications were performed under standard conditions, with a primer-annealing temperature of 55°C. The PCR products were electrophoresed on 1% agarose that contained ethidium bromide. The DNA bands were cut from the gel and purified with the MERmaid spin kit (BIO101).
For direct sequencing, purified DNA served as a template in a sequencing-PCR reaction using the BigDye Terminator Cycle Sequencing Ready Reaction kit, according to the manufacturer’s guidelines (PE Applied Biosystems). Reaction products were ethanol-precipitated, resuspended in formamide loading buffer, and electrophoresed on an ABI 377 automated sequencer.
For restriction-enzyme digestion, the purified DNA was incubated for 3 h at 37°C with the AciI restriction enzyme, according to the manufacturer’s recommendations (New England Biolabs). The digestion products were then electrophoresed on a 1% agarose gel and stained with ethidium bromide.
Tissue Analysis
Tonsil and adenoid tissue samples were processed and studied according to standard procedures for light and electron microscopy (Martin et al. 1975).
Results
Fibroblast Sialic Acid
The cultured fibroblasts of patient 1 were harvested at the confluent-monolayer–culture stage on the third day after culture-medium exchange. The free NeuAc concentration was 36 nmol/mg protein (control <0.1 nmol/mg protein). The free NeuAc in the supernatant culture medium was 13.6 nmol/mL (control <0.1 nmol/mL) and 7.4 nmol/mL in the PBS rinse before scraping the cells. The subcellular distribution of free NeuAc in the patient’s fibroblasts was characteristic of sialuria. Although 81% of the total amount of the lysosomal marker enzyme (hexosaminidase) was present in the lysosomal fraction, only 21% of the recovered NeuAc was found in the lysosomes, and 59% of the recovered NeuAc was in the soluble fraction representing the cytoplasm (table 1).
Table 1.
Distribution of Free Sialic Acid in Subcellular Fractions of Fibroblasts
|
% of Total Free Neuraminic Acid Recovered from |
|||||
| Fraction | % of Total Hexosaminidasea Recovered from Patient 1 | Patient 1 | Control Patientsb | Patients with Sialuriab,c | Patients with ISSDc,d |
| Nuclear | 16 | 13 | 18 | 5, 7 | 5, 6 |
| Lysosomal | 81 | 21 | 21 | 4, 3 | 56, 77 |
| Microsomal | 3 | 7 | 3 | 2, 2 | 29, 80 |
| Soluble | 0 | 59 | 54 | 89, 88 | 10, 10 |
Tissue Analysis
In the proband’s tonsillar tissue, the concentration of free sialic acid was 66.8 nmol/mg protein (control 5.6 nmol/mg protein). Histologically, the tonsil and adenoid tissues showed no storage phenomena, such as foam cells or swollen lysosomal inclusions. These findings were confirmed by electron microscopy, which did not reveal any morphologic abnormality in the mitochondria or the endoplasmic reticulum (data not shown). The latter structures were found to be abnormal in the hepatocytes of two patients with sialuria who were reported elsewhere (Fontaine et al. 1968; Wilcken et al. 1987).
Biochemical and Molecular Diagnoses
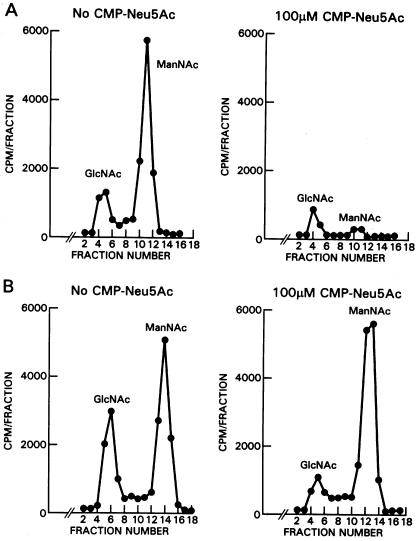
The diagnosis of sialuria was confirmed on both biochemical and molecular grounds. First, enzymatic activity of UDP-GlcNAc 2-epimerase was measured in cultured fibroblasts in the presence and absence of 100 μM CMP-Neu5Ac. Although the normal fibroblast epimerase activity was 95% inhibited by 100 μM CMP-Neu5Ac (fig. 3A), the epimerase of patient 1 was not inhibited at all (fig. 3B). Published levels of inhibition are 79%–100% for normal fibroblasts and 19%–27% for sialuria cells (Seppala et al. 1991; Ferreira et al. 1999).
Figure 3.
CMP-Neu5Ac inhibition of UDP-GlcNAc 2-epimerase activity. The substrate of epimerase is [3H]UDP-GlcNAc, and the product is [3H]ManNAc. [3H]GlcNAc represents a degradation product of [3H]UDP-GlcNAc, not a product of epimerase (Seppala et al. 1992). A, Epimerase activity in normal fibroblasts. Significant ManNAc is formed in the absence of CMP-Neu5Ac (left), and 100 mM CMP-Neu5Ac inhibits ManNAc formation by 95% (right). B, Fibroblast epimerase activity in patient 1. CMP-Neu5Ac, 100 mM (right), did not inhibit ManNAc production at all compared with ManNAc production in the absence of CMP-Neu5Ac (left).
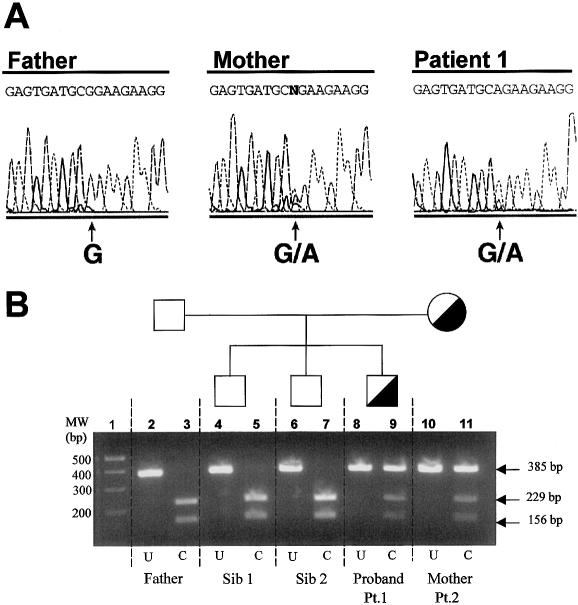
Second, the patient’s epimerase gene was sequenced and found to contain a heterozygous G→A change in nucleotide 848 (fig. 4A). The resulting glutamine-for-arginine substitution in codon 266 has been reported as the causative mutation in sialuria for two of the five patients reported elsewhere (Ferreira et al. 1999; Seppala et al. 1999).
Figure 4.
Molecular diagnosis of sialuria in the family of patient 1. A, Genomic DNA sequencing of patient 1, his father, and his mother (patient 2). At position 848, the father has the normal G, but both the patient and his mother are heterozygous for the G→A substitution. B, Agarose gel electrophoresis of reaction products formed by AciI restriction-enzyme digestion of a 385-bp fragment that includes nucleotide 848. Lane 1, molecular weight markers. Lanes 2 and 3, father’s DNA, uncut (U) and completely cut (C) into 229-bp and 156-bp fragments. Lanes 4–7, DNA from the two unaffected siblings, uncut (lanes 4 and 6) and completely cut (lanes 5 and 7). Lanes 8–11, DNA from the proband (patient 1) and his mother (patient 2), uncut (lanes 8 and 10) and cut (lanes 9 and 11). The cut DNA shows heterozygosity for the normal sequence (229-bp and 156-bp fragments) and the mutation (uncut 385-bp fragment).
Family Studies
It has been proposed, because of the apparent clinical normalcy of the patients' parents, that sialuria is an autosomal dominant disorder and that affected individuals represent new mutations (Weiss et al. 1989; Seppala et al. 1991, 1999). We attempted to confirm this by performing molecular analysis of the UDP-GlcNAc 2-epimerase gene of the DNA in the parents of patient 1. The results indicate that the father has two normal epimerase alleles, but the mother bears the same mutation as her affected son (fig. 4A).
To verify this finding, we made use of the fact that the G848A mutation deletes an AciI restriction site. In normal controls, the 385-bp fragment produced by PCR amplification of the region will be cut into 229-bp and 156-bp fragments. In contrast, the 385-bp fragment is retained if the G848A mutation is present. This restriction-enzyme analysis revealed that the patient and his mother, but not his two unaffected brothers or his father, carry the heterozygous mutation (fig. 4B).
Discussion
Several pieces of evidence indicate that sialuria represents a mild disorder whose manifestations overlap the range of normal. First, patient 1, who is only the sixth case of “French type” sialuria to be described, displayed a very mild course between the ages of 10 and 48 mo. Despite frequent infections in the first 2 years of life, his primary feature was mild delay of neuromotor development. The coarse facial features observed in infancy (fig. 1A) were transient. Patient 1 remained normocephalic, maintained normal joint mobility, and had only equivocal hepatomegaly. Except for its delayed ossification, the skeleton showed no radiographic abnormalities after the boy was 2 years old. The mild hypotonia persisted, but patient 1 was an alert child who made slow but steady progress in speech development.
Descriptions published elsewhere of the five known patients with sialuria (table 2) provide an additional rationale for considering sialuria a mild disease. In infancy, sialuria presents only with nonspecific signs and symptoms, some of which are reminiscent of a lysosomal storage disorder. In each recorded case, the pregnancy was normal, except for one instance of prematurity (Krasnewich et al. 1993). As newborn infants, the patients were small for their gestational age but had normal head circumferences. In general, the patients exhibited a flat, coarse facies and mild developmental delay, which, in one patient, was recorded only as congenital, persistent hypotonia (Wilcken et al. 1987). Hepatomegaly was consistently recorded in all patients but was usually mild and was documented only by clinical examination. Some of the patients experienced frequent upper-respiratory tract infections (UTIs) throughout infancy and into the second year of life, along with episodes of gastroenteritis with dehydration and transient FTT. In two patients, including patient 1 in the present study, prolonged neonatal jaundice was noted. The patient reported here and the original French patient also had microcytic anemia. Children with sialuria have consistently shown delayed skeletal development, with few signs of dysostosis multiplex (fig. 2) in the first years of life. In other respects, physical development has been normal (table 2). Developmental ages or IQs have been borderline low in all patients, and the first child described had moderate retardation. However, later in childhood, neuromotor and mental development have been either mildly impaired or almost unaffected, and two patients attended schools for children without disabilities (Weiss et al. 1989; Krasnewich et al. 1993; Ferreira et al. 1999). Therefore, some older patients may never come to medical attention. Frank seizures, which were controlled with barbititurates, were observed in two instances (Fontaine et al. 1968; Wilcken et al. 1987), and the Portuguese girl with sialuria had hyperthermic convulsions (Ferreira et al. 1999).
Table 2.
Clinical Features of Children with “French Type” Sialuria and Their Mutation in the Epimerase Gene[Note]
|
Features of |
||||||
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4a | Patient 5b | Patient 6 |
| Year identified | 1968 | 1987 | 1989 | 1993 | 1999 | this report |
| Sex | Male | Female | Male | Male | Female | Male |
| Referencec | A | B | C | D | E | F |
| Pre- and perinatal: | ||||||
| Normal pregnancy | + | + | + | + | + | − |
| Normal delivery | + | + | + | + | + | − |
| Gestation (weeks) | 36 | 39 | 40 | 30 | 40 | 35 |
| Anthropometry at birth: | ||||||
| Weight (g) | 2,650 | 2,740 | … | 1,700 | 2,650 | 2,350 |
| Length (cm) | … | … | … | … | 46 | 44 |
| OFC (cm) | … | 34.5 | … | … | 33 | 33 |
| Signs and symptoms: | ||||||
| Age at examination | 4.5 mo | Infancy | 2 mo | Infancy | Infancy | Infancy |
| Developmental delay | + | + | − | + | + | + |
| Hypotonia | … | + | − | … | … | + |
| Coarse facies | + | + | + | + | + | + |
| Hepatomegaly | + | + | + | + | + | − |
| Prolonged jaundice | − | + | … | … | … | + |
| Hypochromic anemia | + | … | … | … | … | + |
| Seizures | + | − | … | + | + | − |
| Transient FTT | + | + | … | … | − | + |
| Frequent UTIs | + | + | … | … | − | + |
| Physical development: | ||||||
| Age at examination (years) | 3.5 | 7.0 | 5.5 | 4.5 | 7.42 | 4.08 |
| Weight (%)d | 10 | 10–25 | … | 25–50 | 75–90 | 10–25 |
| Height (%)d | 3–10 | 10–25 | 25–50 | 25–50 | 75–90 | 10–25 |
| OFC (%)d | … | 97 | … | 25–50 | 50–75 | 25–50 |
| Neuromental assessment: | ||||||
| Age at assessment (years) | 4.17 | 7 | 5.5 | 4.5 | 7.42 | 4.08 |
| Developmental age or IQ | 1.42 years | 4.58 years | Normal | 68 | 78 | 70 |
| Mutation: | ||||||
| Nucleotide change: sitee | … | C→T: 849 | G→A: 848 | G→T: 839 | G→A: 848 | G→A: 848 |
| Amino acid substitutionf | … | R266W | R266Q | R263L | R266Q | R266Q |
Note.— A plus sign (+) indicates presence, and a minus sign (−) indicates absence.
Additional clinical features were mild thoracic scoliosis, enlarged cerebral ventricles, hirsutism, and obstructive sleep apnea.
Additional clinical features were obstructive sleep apnea and febrile seizures.
“A” indicates Fontaine et al. 1968. “B” indicates Wilcken et al. 1987; Don et al. 1991; Seppala et al. 1999. “C” indicates Weiss et al. 1989; Seppala et al. 1991; Gahl et al. 1996; “D” indicates Krasnewich et al. 1993; Seppala et al. 1999. “E” indicates Ferreira et al. 1999. “F” indicates Leroy et al. 1998.
Numbers represent percentile in cumulative chart of physical development.
Nucleotide number according to Genbank accession number NM_005476.
R indicates arginine, W indicates tryptophan, Q indicates glutamine, and L indicates leucine.
Finally, the concept that sialuria represents a mild condition after early childhood is supported by the diagnosis in the proband’s mother (patient 2). This woman—the seventh example and the first adult patient known to have sialuria—was diagnosed retrospectively and did not consider herself affected in any way. Clearly, additional detailed reports are required to definitively delineate the average and the extreme clinical phenotypes of sialuria. It is possible that the phenotypes of patients 1 and 2 are not significantly different, given the disparate social and educational expectations of the son’s and the mother’s generations.
In patient 1, the diagnosis of sialuria was confirmed by the findings of increased urinary excretion of free NeuAc, typical cytoplasmic distribution of free sialic acid within cultured fibroblasts, and reduced inhibition of fibroblast UDP-GlcNAc 2-epimerase by CMP-Neu5Ac, as well as the detection of a mutation (in the putative allosteric domain of the UDP-GlcNAc 2-epimerase gene) that is known to cause sialuria. Specifically, the urinary level of free NeuAc was 167 μmol/mg creatinine, which is slightly above the levels in other patients with sialuria (range 19–117 μmol/mg creatinine) (Ferreira et al. 1999). However, at 4 years and 8 mo of age, the level was found to be 102 μmol/mg creatinine, which is well within the range of values in other patients with sialuria. The same conclusion holds for the urinary excretion of less than half the quantity (48 μmol/mg creatinine) in the mother. At present, no factors are known that may be correlated directly with the amount of free sialic acid in the patients’ urine. Even the confirmation that age may account for some of the fluctuation must await prospective studies in more patients. Similarly, the fibroblast level of free NeuAc (36 nmol/mg protein) was typical of that reported in sialuria cells (17–273 nmol/mg of protein), and the 59% cytoplasmic distribution was also representative of that for patients with sialuria (Ferreira et al. 1999). In sialuria, 50–100 μM CMP-Neu5Ac inhibits UDP-GlcNAc 2-epimerase by 19%–27% (normal 79%–100%), and, in the proband in the present study, the inhibition was zero (fig. 3B). Finally, mutation analysis of the UDP-GlcNAc 2-epimerase gene of the proband in the present study revealed the same R266Q mutation (fig. 4) that was observed in two other patients with sialuria (table 2).
Other diagnoses were systematically eliminated. The finding of an increased level of sialic acid in the urine argued against consideration of other storage disorders, such as the mucopolysaccharidoses or the glycoproteinoses. Deficiency of sialidase, or neuraminidase, was ruled out because the increased NeuAc was free and not bound in the form of glycoconjugates. Disorders of free sialic acid storage were more strongly considered. The increased urinary excretion of free NeuAc was consistent with the diagnosis of infantile free sialic acid–storage disease (ISSD) (MIM 269920). However, patients with this progressive encephalopathy, which results from faulty egress of free sialic acid from lysosomes (Weiss et al. 1989; Gahl et al. 1996), experience frank regression of neuromotor and mental development, alarming neurologic signs, and, occasionally, death in infancy. Moreover, in ISSD, the free NeuAc accumulates in cellular lysosomes rather than in the cytoplasm (Tietze et al. 1989). Patients with Salla disease (MIM 604369), a milder allelic variant of ISSD that is most often observed in Finland, have a later clinical onset and a more chronic course; the excess of free sialic acid in urine is also less pronounced (Gahl et al. 1996). The diagnosis of Salla disease was ruled out by the proband’s mild clinical course, by the lack of a lysosomal abnormality in his lymphoid tissue, and by the cytoplasmic location of the storage of free NeuAc in fibroblasts.
In addition to the progress in its clinical delineation, the observation and study of the two patients with sialuria reported here contribute significantly to current scientific understanding of the disorder. First, the diagnosis of sialuria in the proband’s mother—which was verified molecularly, as well as on the basis of vastly increased urinary excretion of free NeuAc—confirms the dominant mode of inheritance of this disorder. This is in agreement with the demonstration in this and other patients (Cardo et al. 1985; Seppala et al. 1999) that only one of the two alleles of the UDP-GlcNAc 2-epimerase gene contains a mutation. It also represents the first instance in which the disorder in a patient with sialuria is not simply assumed to be the result of a new mutation. Dominant inheritance in sialuria is supported somewhat by the lack of parental consanguinity in every instance reported, and it has a logical basis in the biochemistry of the disorder. In sialuria, the mutant UDP-GlcNAc 2-epimerase retains normal catalytic activity; however, unlike the normal enzyme, it is no longer inhibitable by CMP-NeuAc binding (Kornfeld et al. 1964; Sommar and Ellis 1972; Cardo et al. 1985; Thomas et al. 1985; Seppala et al. 1999) (fig. 3). Thus, even in the heterozygous state, the mutant allele produces a constitutively active, rate-limiting enzyme that achieves sufficient NeuAc synthesis to cause overproduction of free sialic acid. A similar, recently documented example of an inborn error of metabolism with dominant inheritance due to failed allosteric inhibition is the syndrome of hyperinsulinism and hyperammonia in infants who are heterozygous for a mutation in the allosteric domain of the glutamate dehydrogenase gene (Stanley et al. 1998).
The failure of allosteric regulation of UDP-GlcNAc 2-epimerase may not be the sole explanation for the dysregulated synthesis of free NeuAc in sialuria. Enhanced stability of the enzyme may also contribute to overproduction of NeuAc (Ferreira et al. 1999). There is no evidence suggesting this possibility, however, and the failed feedback inhibition of UDP-GlcNAc 2-epimerase activity amply explains the excessive urinary excretion, the significantly increased levels of NeuAc in the mutant cultured fibroblasts and in lymphoid tissue, and the intracellular distribution of NeuAc primarily in the cytoplasm, as opposed to its intralysosomal accumulation in ISSD cells (table 1).
A second conclusion to be drawn from the proband’s family is that the G→A transition in the second nucleotide of codon 266 probably represents a real mutation, not merely a polymorphism. The mutation is exactly the same as that found in two of the five other patients with sialuria, and it results in a glutamine-for-arginine missense substitution (Ferreira et al. 1999; Seppala et al. 1999). Furthermore, in the family reported here, the mutation segregates with the disorder (fig. 4B), as indicated by increased urinary excretion of free sialic acid observed only in the two family members who bear the mutation. Other reported patients are heterozygous for a C→T transition in the third nucleotide of codon 266, resulting in an arginine-to-tryptophan substitution, or a G→T transversion in the second base of codon 263, causing an arginine-to-leucine substitution (Seppala et al. 1999) (table 2). The clustering of all these mutations in the region of codons 263–266 supports the hypothesis that this stretch of amino acids in the aminoterminal part of the epimerase gene product is part of the allosteric site for CMP-Neu5Ac binding and its normal inhibitory feedback action.
Finally, we believe that the prevalence of sialuria may be underestimated because of its mild phenotype and the variable increase in NeuAc excretion. Moreover, assay of urinary levels of free NeuAc is not a routine laboratory procedure and is generally performed only for confirmation or exclusion of the diagnosis of ISSD or Salla disease in infants or toddlers with progressive brain disease. We note that the mother of our patient was never clinically suspected of having sialuria. Consequently, the immediate relatives of known patients with sialuria should be screened for mutations in codons 263–266 of the UDP-GlcNAc 2-epimerase gene. In addition, assay of urinary level of free NeuAc could be part of the metabolic screening of young children with mild developmental delay.
Acknowledgments
We gratefully acknowledge the work on the DNA extractions from peripheral blood samples of proband and family, which was performed in the division of molecular biology (headed by Dr. Ludwine Messiaen), Department of Medical Genetics (Dr. Anne De Paepe, chairperson), Ghent University School of Medicine, Ghent, Belgium. We also gratefully acknowledge the support for this project to the first author during a sabbatical leave at the Greenwood Genetic Center, Greenwood, SC, and at the Baylor College of Medicine, Department of Molecular and Human Genetics, Houston.
Electronic-Database Information
Accession number and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Web/Genbank/index.html (for accession number NM-005476)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for sialuria [MIM 269921])
References
- Aula P, Gahl WA (2001) Disorders of free sialic acid storage. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th ed. McGraw-Hill, New York, pp 5109–5120 [Google Scholar]
- Cardo PP, Lombardo C, Gatti R (1985) A simple detection of sialic acid storage disorders by urinary “free” and “total” sialic acid determinations. Clin Chim Acta 150:129–135 [DOI] [PubMed] [Google Scholar]
- Don NA, Wilcken B (1991) Sialuria: a follow-up report. J Inher Metab Dis 14:942 [DOI] [PubMed] [Google Scholar]
- Ferreira H, Seppala R, Pinto R, Huizing M, Martins E, Braga AC, Gomes L, Krasnewich DM, Sa Miranda MC, Gahl WA (1999) Sialuria in a Portuguese girl: clinical, biochemical and molecular characteristics. Mol Genet Metab 67:131–137 [DOI] [PubMed] [Google Scholar]
- Fontaine G, Biserte G, Montreuil J, Dupont A, Farriaux JP (1968) La sialurie: un trouble métabolique original? Helv Paediatr Acta (Suppl) 17:1–32 [PubMed] [Google Scholar]
- Gahl WA, Krasnewich DM, Williams JC (1996) Sialidosis In: Moser HW (ed) Handbook of clinical neurology. Vol 22. Neurodystrophies and neurolipidoses, Elsevier, Amsterdam, pp 353–375 [Google Scholar]
- Kornfeld S, Kornfeld R, Neufeld EF, O’Brien PJ (1964) The feedback control of sugar nucleotide biosynthesis in liver. Proc Natl Acad Sci USA 52:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnewich DM, Tietze F, Krause W, Pretzlaff R, Wenger DA, Diwadkar V, Gahl WA (1993) Clinical and biochemical studies in an American child with sialuria. Biochem Med Metab Biol 49:90–96 [DOI] [PubMed] [Google Scholar]
- Leroy JG, Dacremont G, Desimpel H, Van Coster R (1998) Sialuria (French type): new observation of this rare disorder. Am J Hum Genet Suppl 63:A269 [Google Scholar]
- Leroy JG, Ho MW, MacBrinn MC, Zielke K, Jacob J, O’Brien JS (1972) I-cell disease: Biochemical studies. Pediatr Res 6:752–759 [DOI] [PubMed] [Google Scholar]
- Martin JJ, Leroy JG, Farriaux JP, Fontaine G, Desnick RJ, Cabello A (1975) I-cell disease (Mucolipidosis II): a report on its pathology. Acta Neuropathol (Berl) 33:285–305 [DOI] [PubMed] [Google Scholar]
- Montreuil J, Biserte G, Strecker G, Spik G, Fontaine G, Farriaux JP (1968) Description d’un nouveau type de méliturie: la sialurie. Clin Chim Acta 21:61–69 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- Seppala R, Lehto VP, Gahl WA (1999) Mutations in the human UDP-N-acetylglucosamine 2-epimerase gene define the disease sialuria and the allosteric site of the enzyme. Am J Hum Genet 64:1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala R, Tietze F, Krasnewich D, Weiss P, Ashwell G, Barsh G, Thomas GH, Packman S, Gahl WA (1991) Sialic acid metabolism in sialuria fibroblasts. J Biol Chem 266:7456–7461 [PubMed] [Google Scholar]
- Sommar KM, Ellis DB (1972) Uridine diphosphate N-acetyl-d-glucosamine 2-epimerase from rat liver: catalytic and regulatory properties. Biochim Biophys Acta 268:581–589 [DOI] [PubMed] [Google Scholar]
- Stanley CA, Lieu YK, Hsu BYL, Burlina AB, Greenberg C, Hopwood N, Perlman K, Rich BH, Zammarchi E, Poncz M (1998) Hyperinsulinism and hyperammonia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 338:1352–1357 [DOI] [PubMed] [Google Scholar]
- Thomas GH, Reynolds LW, Miller CS (1985) Overproduction of N-acetylneuraminic acid (sialic acid) by sialuria fibroblasts. Pediatr Res 19:451–455 [DOI] [PubMed] [Google Scholar]
- Tietze F, Seppala R, Renlund M, Hopwood JJ, Harper GS, Thomas GH, Gahl WA (1989) Defective lysosomal egress of free sialic acid (N-acetyl-neuraminic acid ) in fibroblasts of patients with infantile free sialic storage disease. J Biol Chem 264:15316–15322 [PubMed] [Google Scholar]
- Weiss P, Tietze F, Gahl WA, Seppala R, Ashwell G (1989) Identification of the metabolic defect in sialuria. J Biol Chem 264:17635–17636 [PubMed] [Google Scholar]
- Wilcken B, Don N, Greenaway R, Hammond J, Sosula L (1987) Sialuria: a second case. J Inher Metab Dis 10:97–102 [DOI] [PubMed] [Google Scholar]