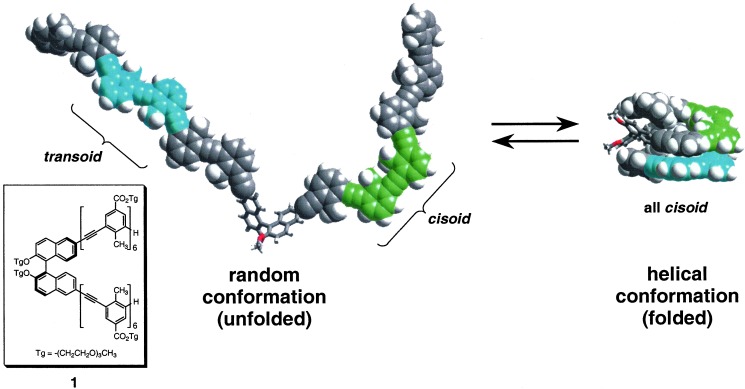
Figure 1.
The chemical structure of 1: a bis-hexameric phenylene ethynylene tethered through (R)-binaphthol (Inset); the side chains promote solubility in a wide range of solvents. The solvent-dependent folding reaction of 1: the unfolded state contains both transoid and cisoid backbone conformations, which become all-cisoid in the folded state. The result is a helical conformation stabilized by intramolecular aromatic–aromatic contacts whose strength is modulated by solvent. The (R)-binaphthol moiety induces a twist sense bias in the resulting helix. Side chains have been omitted for clarity. The helical conformation shown is an energy minimized structure.§